Copyright
©The Author(s) 2016.
World J Biol Chem. Feb 26, 2016; 7(1): 178-187
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.178
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.178
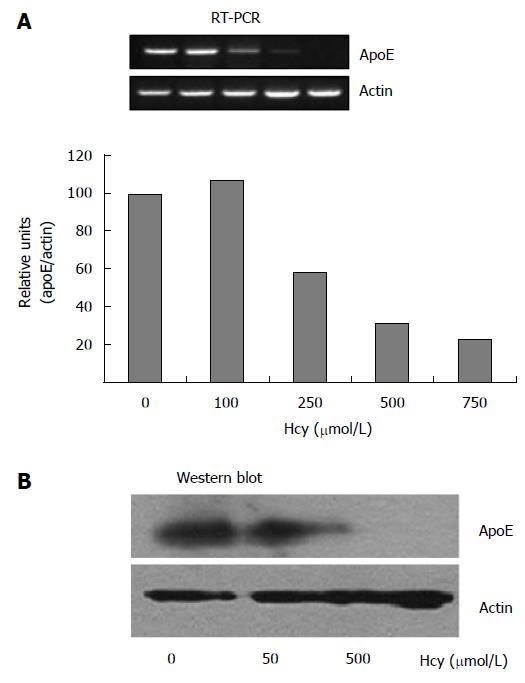
Figure 1 High homocysteine doses downregulate apoE expression in HEK-293 cells.
ApoE expression in HEK-293 cells exposed for 24 h to increasing concentrations of homocysteine (50-750 μmol/L) was assessed by RT-PCR and immunoblotting. A: High homocysteine concentrations (250-750 μmol/L) significantly decreased apoE mRNA levels in HEK-293 cells; in contrast, lower doses of homocysteine (100 μmol/L), did not affect the expression level as compared to the control cells. The expression of β-actin was used for normalization; B: Treatment of HEK-293 cells with 500 μmol/L homocysteine, but not with 50 μmol/L homocysteine, dramatically reduced apoE protein expression. RT-PCR: Reverse transcriptase polymerase chain reaction; ApoE: Apolipoprotein E.
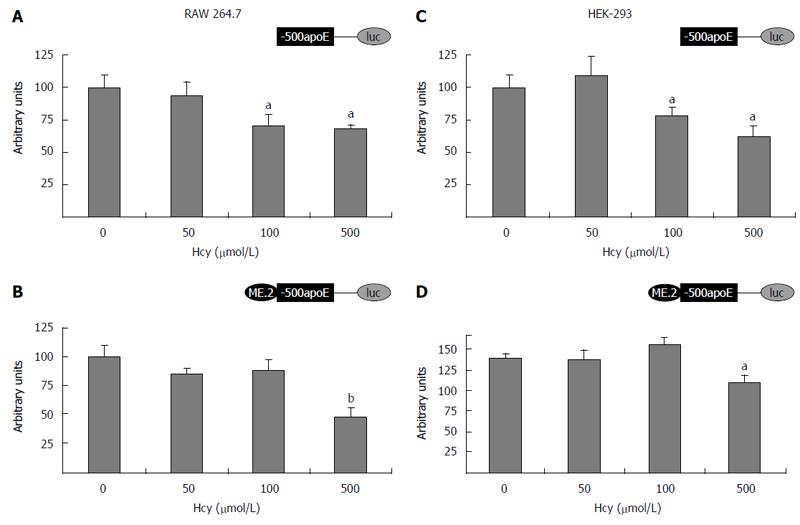
Figure 2 Homocysteine inhibits the activity of apolipoprotein E proximal promoter in either absence or presence of ME2 in RAW 264.
7 macrophages and HEK-293 cells. Transient transfection experiments were performed using (-500/+73) apoE proximal promoter (A, C) or ME2/(-500/+73) multienhancer/apoE promoter constructs (B, D) driving the luciferase reporter and the transfected cells were incubated with 50-500 μmol/L homocysteine for 24 h. Then, luciferase activity was assessed and normalized to the activity of the co-transfected β-galactosidase. Treatment of RAW 264.7 (A, B) and HEK-293 cells (C, D) with 500 μmol/L Hcy significantly decreased (aP < 0.05 or bP< 0.01) apoE promoter activity, in the presence (B, D) or in the absence of ME2 (A, C). Low concentrations of Hcy (50 μmol/L) did not considerably decrease (P > 0.05) the activity of apoE promoter either in the absence (A, C) or in the presence of ME2 (B, D). MEK1/2: MAPK/ERK kinase; Hcy: Homocysteine; ApoE: Apolipoprotein E.
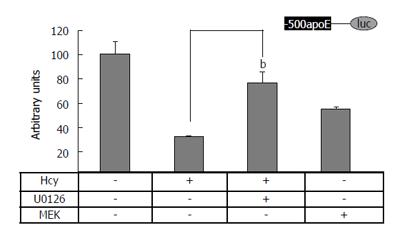
Figure 3 MEK1/2 is a downstream effector of Hcy action on apolipoprotein E promoter in HEK-293 cells.
Transient transfections in HEK-293 cells were performed using plasmids containing apoE proximal promoter (-500/+73 region) in the presence or in the absence of homocysteine alone or in combination with U0126 (a MEK inhibitor), or MEK1 expression vectors. Hcy treatment as well as MEK1 overexpression significantly decreased (P < 0.05) the activity of (-500/+73) apoE-luc construct, as compared to the control. The decreased activity of the reporter luciferase gene driven by the apoE proximal promoter (-500/+73) in the presence of 500 μmol/L homocysteine was partially restored by 10 μmol/L U0126 (bP < 0.01). MEK1/2: MAPK/ERK kinase; Hcy: Homocysteine; ApoE: Apolipoprotein E.
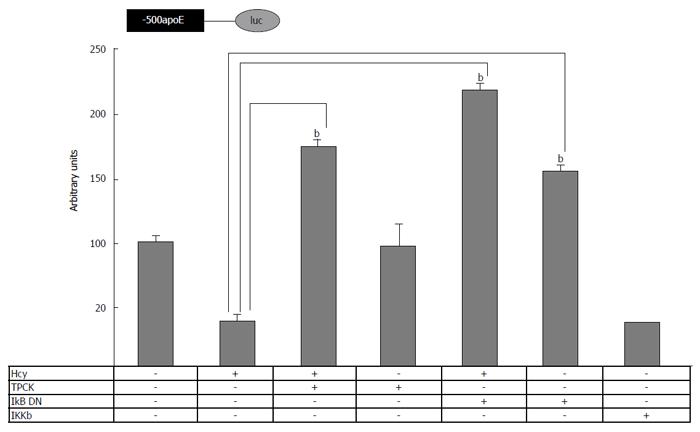
Figure 4 Involvement of nuclear factor kappa B signaling pathway in homocysteine-mediated inhibition of apolipoprotein E promoter activity.
HEK-293 cells were transiently transfected with plasmids containing apoE proximal promoter in the presence of homocysteine, TPCK or expression vectors for IKβ dominant negative (IKβ DN) or IKKβ. The activity of the luciferase reporter, normalized to the co-transfected β-galactosidase, was significantly reduced (P < 0.05) by IKKβ overexpression, similarly to Hcy treatment. TPCK (NF-κB inhibitor) as well as IKβ DN overexpression abrogated the inhibitory effect of homocysteine on apoE promoter activity, bP < 0.001. NF-κB: Nuclear factor kappa B; TPCK: N-p-Tosyl-L-phenylalanine chloromethyl ketone; Hcy: Homocysteine; IKKβ: IκB kinase β; ApoE: Apolipoprotein E.
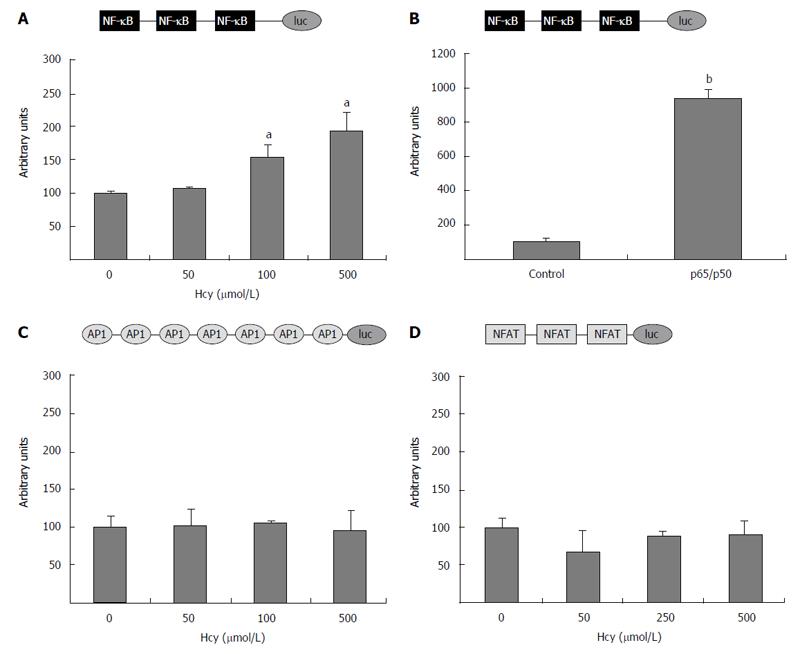
Figure 5 Homocysteine activates nuclear factor kappa B, but has no effect on activator protein-1 or nuclear factor of activated T cells activity.
HEK-293 cells were transiently transfected with (NF-κB)3-luc construct (A), (AP-1)7-luc (C), or (NFAT)3-luc (D) and exposed to homocysteine (50-500 μmol/L). For positive control, the cells were co-transfected with (NF-κB)3-luc and the vector for the heterodimer p65/50. The activity of the luciferase reporter driven by the corresponding promoter was normalized to the co-transfected β-galactosidase. Treatment with high concentrations of homocysteine (250 and 500 μmol/L) significantly increased (aP < 0.05) the activity of the promoter containing the three NF-κB binding sites, while no effect of Hcy (P > 0.05) was observed on promoters containing binding sites for AP-1 (C) or NFAT (D). The activity of the (NF-κB)3-luc construct was greatly enhanced by p65/p50 overexpression, used as positive control (bP < 0.001). NF-κB: Nuclear factor kappa B; AP-1: Activator protein-1; NFAT: Nuclear factor of activated T cells; Hcy: Homocysteine.
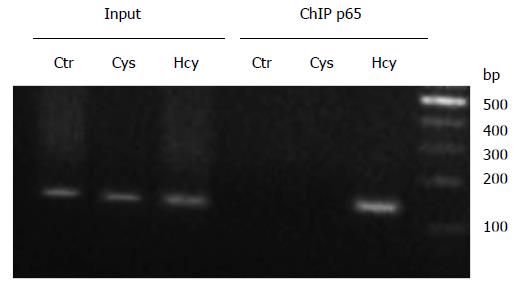
Figure 6 Nuclear factor kappa B p65 subunit is recruited to apolipoprotein E promoter following homocysteine treatment.
Chromatin immunoprecipitation experiments were performed in HEK-293 cells treated with 500 μmol/L Hcy or Cys. Treatment with Hcy induced the recruitment of NF-κB p65 subunit to apoE promoter (lane p65 Hcy), while no binding of p65 proteins was observed on the apoE promoter following Cys treatment or in untreated cells (lanes p65 Cys and Ctr, respectively). PCR using the input as template and primers for the apoE promoter resulted in the expected bands (lanes Input Ctr, Cys and Hcy, respectively). NF-κB: Nuclear factor kappa B; ApoE: Apolipoprotein E; Hcy: Homocysteine; Cys: Cysteine.
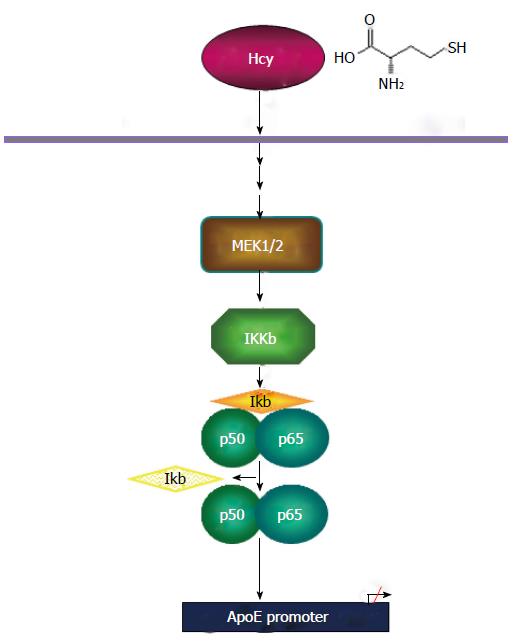
Figure 7 Schematic model of homocysteine-mediated apolipoprotein E gene downregulation.
Homocysteine downregulates apoE expression via MEK1/2 activation, IKKβ and p65/50 subunits of NF-κB. NF-κB: Nuclear factor kappa B; apoE: Apolipoprotein E; IKKβ: IκB kinase β.
- Citation: Trusca VG, Mihai AD, Fuior EV, Fenyo IM, Gafencu AV. High levels of homocysteine downregulate apolipoprotein E expression via nuclear factor kappa B. World J Biol Chem 2016; 7(1): 178-187
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/178.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.178