Copyright
©The Author(s) 2015.
World J Biol Chem. Nov 26, 2015; 6(4): 358-365
Published online Nov 26, 2015. doi: 10.4331/wjbc.v6.i4.358
Published online Nov 26, 2015. doi: 10.4331/wjbc.v6.i4.358
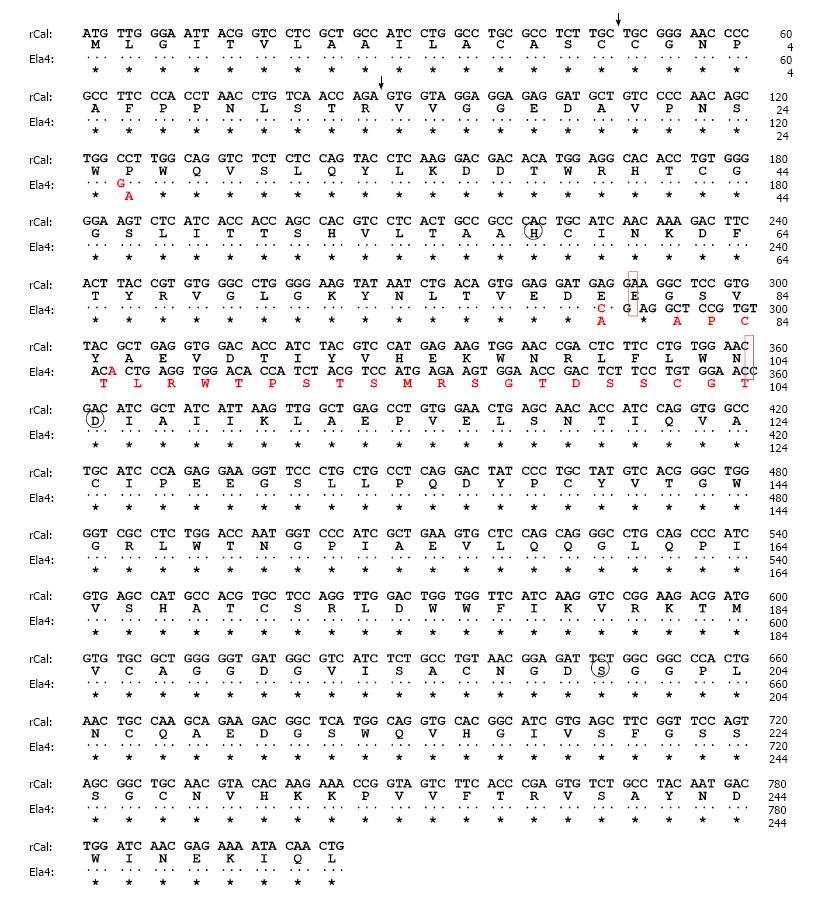
Figure 1 Nucleotide and deduced amino acid sequences of rat caldecrin (rCal) and elastase IV (Ela4).
The nucleotide (upper row) and amino acid (lower row) sequences of the indicated molecules are shown. The dots and asterisks indicate nucleotides and amino acid residues, respectively, that are conserved between rCal and Ela4. Circle: Charge-relay system; Vertical arrowhead: Proteolytic cleavage site.
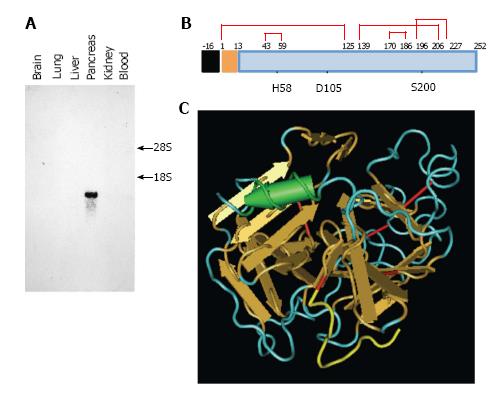
Figure 2 Caldecrin expression and protein structure.
A: Caldecrin expression was analyzed by Northern blot. 18S, 28S: 18S, 28S ribosomal RNA; B: Domain structures of caldecrin. Black box: signal peptide; orange box: pro-peptide; blue box: mature protein; red line: disulfide bridges with cysteine number; the H (histidine), D (aspartic acid), S (serine) catalytic triad; C: Ribbon diagram of the crystal structure of human caldecrin (adapted from PDB ID: 4H4F, prepared from [16]). Red line: Disulfide bridge; Yellow line: Pro-peptide; Arrow: β-sheet structure; Cylinder: α-helix structure.
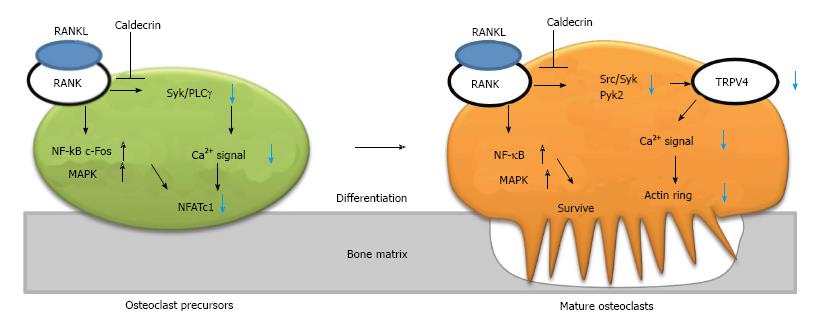
Figure 3 Caldecrin suppresses RANKL-induced osteoclast differentiation and bone resorption.
RANKL binds to its receptor (RANK) on the osteoclast precursor, leading to simultaneous activation of two pathways: the NF-κB/ MAPK/c-Fos axis and the Syk/PLCγ-calcium oscillation- NFATc1 axis. In mature osteoclasts, RANKL also activates the NF-κB/MAPK and Src/Syk/Pyk2-TRPV4 channel–calcium entry–actin ring formation axes. Caldecrin inhibits the latter pathways (but not the NF-κB/MAPK pathway) in the precursor and mature osteoclasts. TRPV4: Transient receptor potential vanilloid channel 4; MAPK: Mitogen-activated protein kinase; RANKL: RANK ligand; PLCγ: Phospholipase Cγ; Pyk2: Proline-rich tyrosine kinase 2; NFATc1: Nuclear factor of activated T-cells cytoplasmic 1; NF-κB: Nuclear factor-kappa B; Syk: Spleen tyrosine kinase.
- Citation: Tomomura M, Tomomura A. Caldecrin: A pancreas-derived hypocalcemic factor, regulates osteoclast formation and function. World J Biol Chem 2015; 6(4): 358-365
- URL: https://www.wjgnet.com/1949-8454/full/v6/i4/358.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i4.358