Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 95-109
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.95
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.95
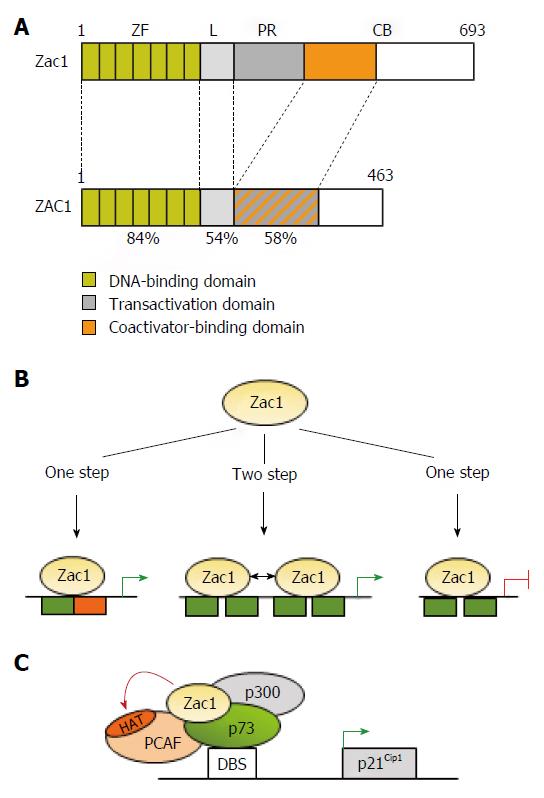
Figure 1 Transcriptional activities of Zac1.
A: Schematic drawing of Zac1 proteins. Domains are boxed and numbers refer to amino acids and percentage of homology between mouse and human. The N-terminal DNA binding domain is highly conserved between human and mice and comprises seven canonical C2H2-type zinc fingers (ZF). The linker domain (L) in conjunction with the proline-rich region (PR) confers transactivation in mice, which is further enhanced through the C-terminus’s coactivator binding (CB) domain. Contrarily, transactivation and coactivator recruitment jointly localize to ZAC1’s C-terminus; B: Zac1 recognizes different DNA-elements to confer transcriptional regulation. Monomeric Zac1 binding to GC-rich palindromes (left) or dimerization at G/C rich direct repeat elements (middle) results in transactivation. On the other hand, monomeric Zac1 binding to G/C rich half sites causes repression (right); C: Zac1 coactivation of p73. Following recognition of its DNA-binding site (DBS), the transcription factor p73 recruits Zac1 together with the general coactivator p300 and PCAF to the p21Cip1 promoter during early neural differentiation. Due to its scaffolding function, Zac1’s zinc fingers stabilize this interaction and enhance additionally PCAF’s histone acetyltransferase (HAT) activity. This event enhances histone acetylation at the p21Cip1 promoter and subsequent transcription.
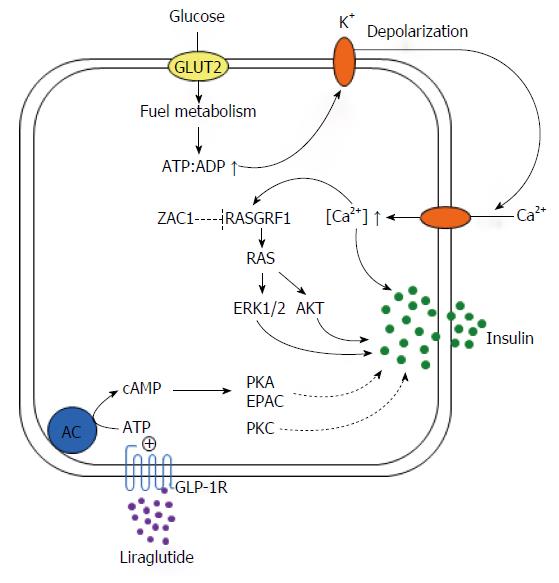
Figure 2 Roles for RAS protein-specific guanine nucleotide-releasing factor 1 in insulin secretion and transient neonatal diabetes mellitus type 1.
Glucose is the main stimulus for insulin secretion. Once transported into pancreatic β-cells by glucose transporter 2 (GLUT2), metabolisation of glucose results in the production of ATP. A subsequent rise in the ATP:ADP ratio drives closure of ATP-regulated K+ channels and accumulation of intracellular K+. Heightened K+ concentrations lead to depolarization of the plasma membrane and promote opening of voltage-dependent Ca2+ channels with subsequent influx of Ca2+ and increases in free cytoplasmatic Ca2+. This event stimulates exocytosis of insulin from the insulin-storing secretory granules through different routes including activation of the Ca2+-calmodulin activated RAS protein-specific guanine nucleotide-releasing factor 1 (RASGRF1), a direct ZAC1 target gene. In its activated state the small G-protein RAS couples to ERK1/2 and PI3K/AKT signaling among other downstream effectors, which jointly enhance the exocytotic process of insulin secretion. ZAC1 overexpression in transient neonatal diabetes mellitus type 1 (TNDM1) is predicted to directly repress RASGRF1 and consequently GSIS. Contrarily, PKA signaling is undisturbed in TNDM1 as well as the potentiating effects of the GLP-1R agonist liraglutide on GSIS.
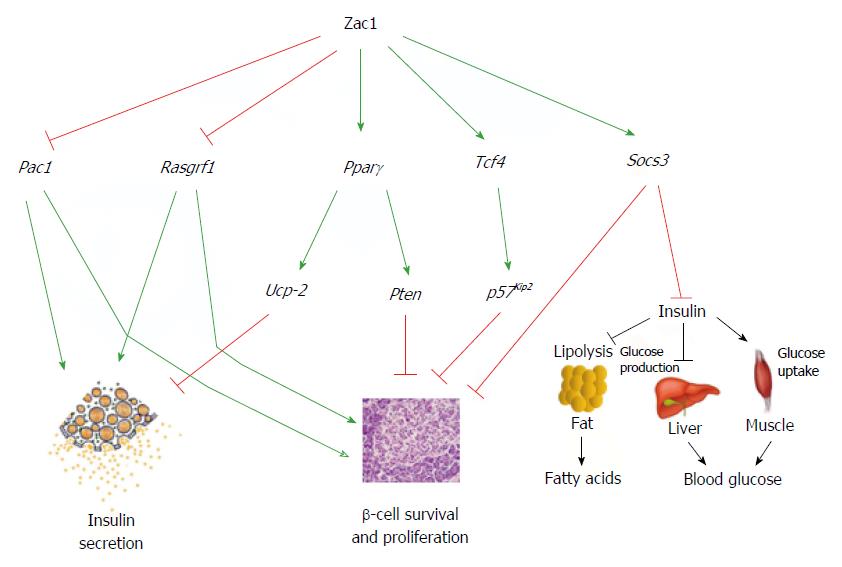
Figure 3 Integrated model of Zac1’s role in β-cell function and glucose metabolism.
Zac1 DNA-binding at downstream target genes confers transcriptional activation or repression in a partly tissue-specific manner in mice. In this respect Zac1 binding represses Pac1 and Rasgrf1 in pancreatic β-cells leading to an impaired insulin-secretory response and GSIS (left). Additionally, Zac1 upregulation of the transcription factors Pparγ and Tcf4 may translate in enhanced expression of their downstream targets Ucp-2, Pten, and p57Kip2 with an inhibitory role in GSIS and β-cell proliferation (middle). Moreover, Zac1-dependent transactivation of Socs3 may attenuate growth factor and insulin signaling in β-cells and peripheral tissues, respectively (right).
- Citation: Hoffmann A, Spengler D. Role of ZAC1 in transient neonatal diabetes mellitus and glucose metabolism. World J Biol Chem 2015; 6(3): 95-109
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/95.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.95