Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 83-94
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.83
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.83
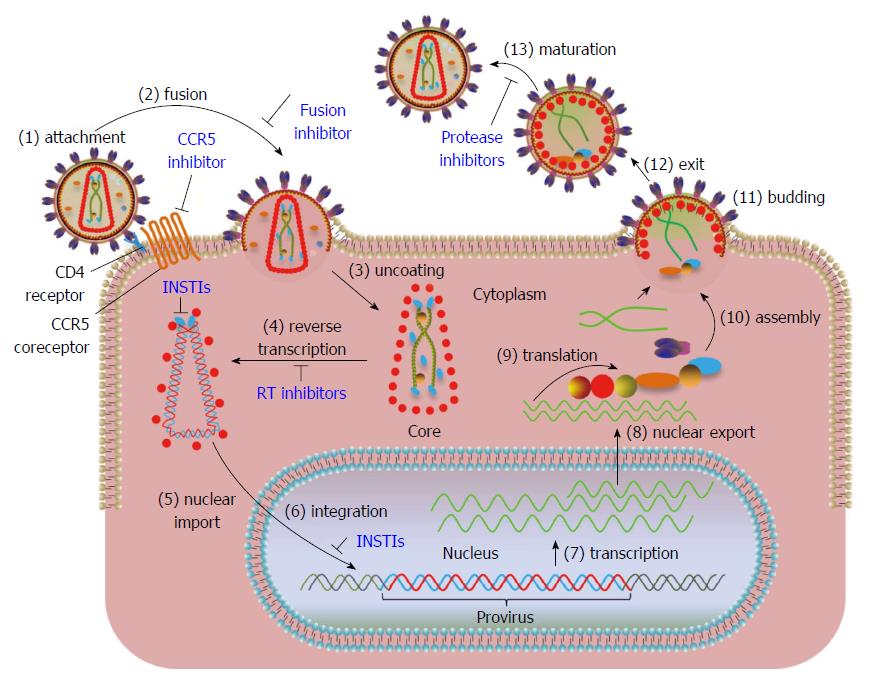
Figure 1 Overview of human immunodeficiency virus type 1 replication cycle.
Steps in the viral replication cycle are numbered. The steps where FDA approved drugs exist are marked in blue. (1) The trimeric envelope glycoprotein (gp120/gp41) of human immunodeficiency virus (HIV) binds to the CD4 receptor and CCR5 co-receptors present on host cell surface. Maraviroc, a CCR5 receptor antagonist blocks the binding of viral gp120 to CCR5 and prevents the entry of virus into cells; (2) Viral membrane fuses with cellular membrane allowing the entry of the virus into the cytoplasm. Enfuvirtide, a fusion inhibitor binds to gp41 and prevents the formation of an entry pore for the capsid of the virus, thus blocking the entry of virus into the cytoplasm; (3) The virus undergoes uncoating of the core; (4) HIV RNA is reverse-transcribed by reverse transcriptase into double stranded DNA within the core. Reverse-transcriptase inhibitors block the synthesis of viral DNA; (5) Integrase (IN) cleaves two nucleotides adjacent to conserved CA nucleotides from both 3’-ends of viral DNA (referred as 3’-processing in text) and forms the preintegration complex (PIC). The PIC is transported into the nucleus through a cellular nuclear import pathway. IN strand transfer inhibitors (INSTIs) bind to the IN-DNA complex after 3’-processing rendering the cytoplasmic PIC defective for integration but not nuclear transport; (6) IN facilitates the joining of viral DNA to the host DNA (termed strand transfer in text). Integrated viral DNA is referred to as “provirus”. A cellular protein, lens-epithelium derived growth factor (LEDGF/p75), preferentially targets the integration of viral DNA within transcriptionally active regions. INSTIs block the joining of processed viral DNA ends into cellular DNA; (7) The viral DNA is transcribed by cellular RNA pol II into viral RNAs; (8) Full-length and alternatively spliced RNAs are transported out of nucleus; (9) Viral proteins are synthesized from the RNA template; (10) Full-length viral RNA and newly synthesized proteins begin the assembly of new virus particles at the inner surface of cell membrane; (11) Immature viral particles bud out of the membrane and released (12); and (13) Proteolytic processing of viral proteins results in the maturation of viral particle. Protease inhibitors block the formation of mature proteins by inhibiting the proteolytic processing of the viral polyproteins.
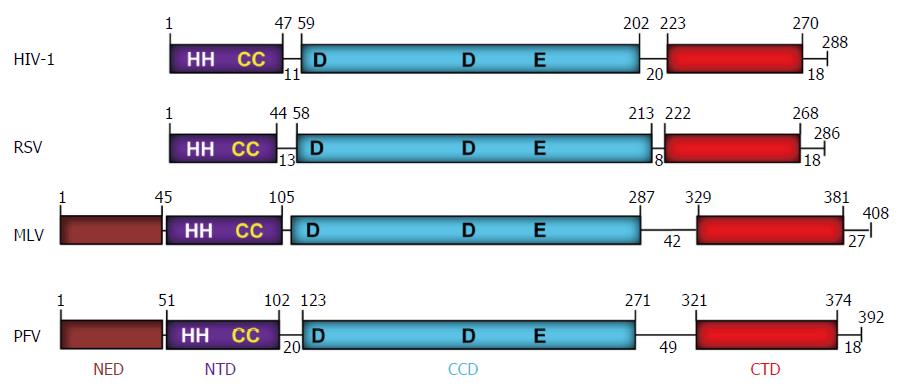
Figure 2 Domain organization of retroviral integrases.
The domains (NED: N-terminal extension domain; NTD: N-terminal domain; CCD: Catalytic core domain; and CTD: C-terminal domain) are depicted with different colors. The number of residues in each domain are indicated. The domains are separated by protein linkers of various sizes. NTD contains the zinc binding HH-CC motif and the D-D-35-E motif in CCD binds Mg2+ which constitutes the active site. The CTD shows a topology of SH3-like domain, a characteristic feature associated with protein-protein and protein-DNA interactions. The exact MLV domain sizes have not been determined experimentally except for the CTD[99]. A short tail of disordered amino acids is located on the C-terminal end of each IN. IN: Integrase; HIV: Human immunodeficiency virus; RSV: Rous sarcoma virus; MLV: Murine leukemia; PFV: Prototype foamy virus.
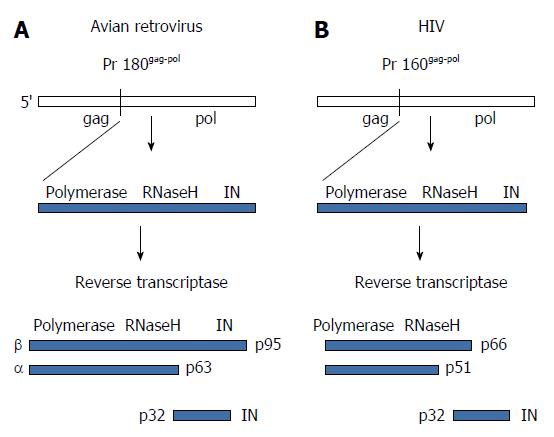
Figure 3 Structural organization and proteolytic processing of reverse transcriptase and integrase.
A: The precursor Gag-Pol protein is processed by the viral protease into an intermediate polyprotein. The avian polyprotein exists as a dimer of the β subunit that is processed into the active αβ subunits (RT) and integrase (IN). The α subunit contains the active polymerase and RNaseH sites while β retains IN residues. RT does not possess integration capabilities; B: Cleavage of the human immunodeficiency virus (HIV) polyprotein produces the active dimeric RT and IN. The HIV p66 subunit possesses the polymerase and RNaseH active sites. RT: Reverse transcriptase.
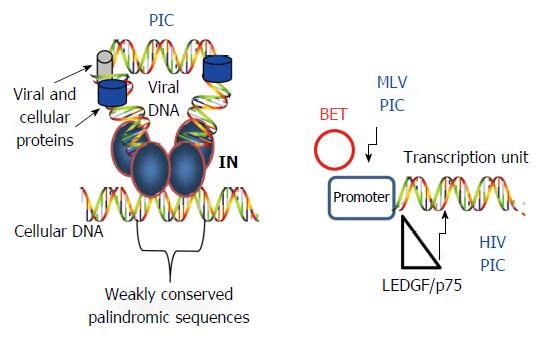
Figure 4 Selection of cellular DNA target sites for integration.
Left: IN within the PIC is guided to host sites by several mechanisms including recognition of weak palindromic sequences by IN, nuclear topography of the cellular host DNA site, chromatin structure, and several kinds of associated histone tails. Only IN (labeled), RT (blue circle) and LEDGF/p75 (gray cylinder) are identified in the figure; Right: Cell proteins (BET and LEDGF/p75) influence the PIC to preferentially insert the viral DNA into specific regions of the host genome. IN: Integrase; PIC: Preintegration complex; HIV: Human immunodeficiency virus; MLV: Murine leukemia virus.
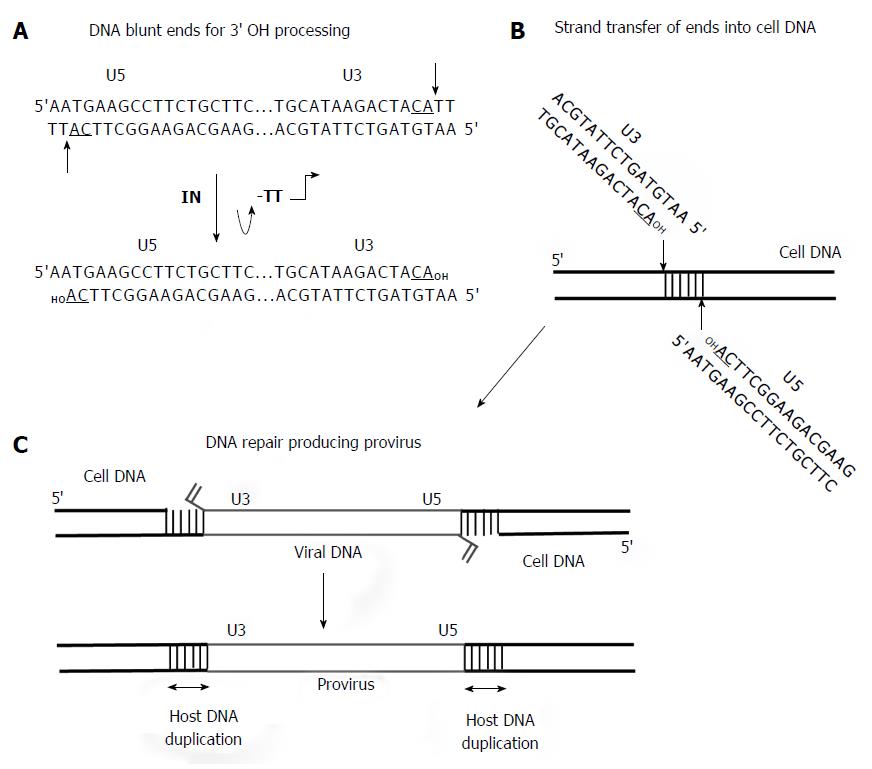
Figure 5 Requirement for 3’ OH processing and strand transfer activities to facilitate concerted integration of viral DNA by integrase into target DNA.
A: The linear approximately 10 kb DNA with only the ends is shown. The U3 and U5 RSV DNA blunt ends with the underlined conserved CA dinucleotide on the catalytic strand are indicated. The thin arrows indicate 3’ OH processing of two nucleotides by IN producing the recessed substrate for strand transfer activity; B: Concerted integration of the viral linear DNA ends by integrase (IN) into the target DNA is by a transesterification mechanism; C: The insertion of viral DNA into the target produces a 6 bp stagger cut by RSV IN that is repaired by cellular proteins producing the integrated provirus. RSV: Rous sarcoma virus.
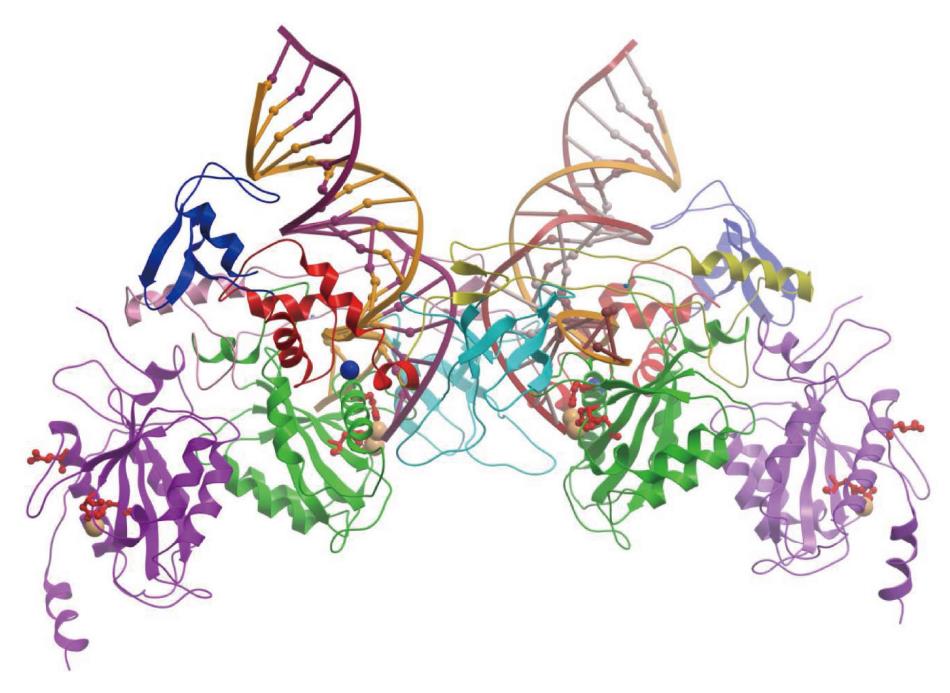
Figure 6 Prototype foamy virus intasome structure.
The intasome consist of an integrase (IN) tetramer with two viral DNA ends. The 17/19 bp DNA interacts extensively with the two inner monomers of IN. Among the inner monomers, the N-terminal extension domain, N-terminal domain, catalytic core domain (CCD) and C-terminal domains are shown in blue, red, green and cyan, respectively. The DDE residues are identified as red amino acids. Among the outer protomers, only the CCD (shown in purple) was resolved. The cartoon was redrawn from the PDB ID 3OY9 in Molsoft ICM-Browser. The Zn2+ and Mg2+ metal ions are shown with blue and orange spheres, respectively.
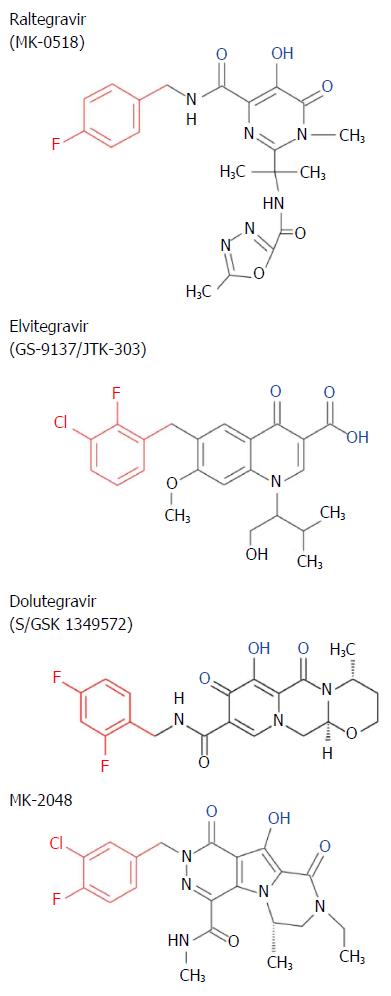
Figure 7 Chemical structure of strand transfer inhibitors.
Conserved metal chelating moiety similar to diketo acid is shown in blue and a conserved halogenated benzyl group that interacts with the penultimate nucleotide of DNA is shown in red.
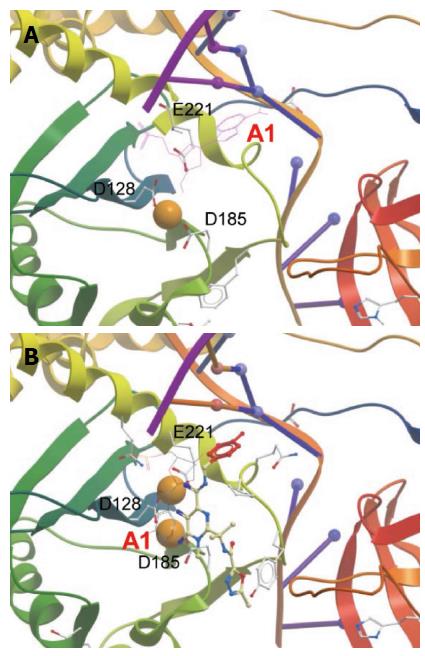
Figure 8 Active site within prototype foamy virus intasome in apo (A) and Raltegravir-bound state (B).
Orange spheres indicate Mg2+. Active sites residues are labeled. Only the terminal 3’ adenosine (marked A1) is displayed in its chemical form to show the displacement upon RAL binding. The diketo acid group of Raltegravir (RAL) interacts with the metal ions. Adenine is π-stacked against the RAL metal-chelating scaffold. The halobenzoyl group of RAL is marked red. The figures were constructed from PDB entries 3L2R and 3OYA, respectively, in Molsoft ICM-Browser.
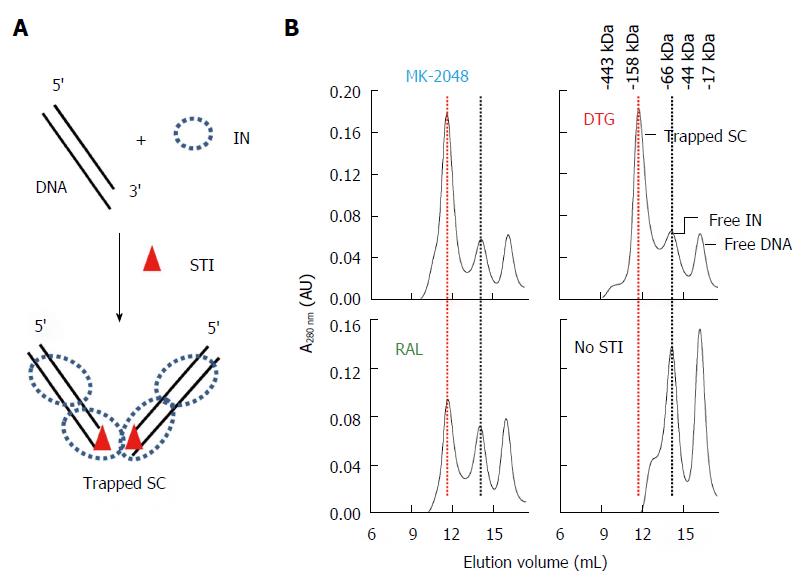
Figure 9 Identification of strand transfer inhibitor-trapped Rous sarcoma virus synaptic complex by size-exclusion chromatography.
A: Schematic of Rous sarcoma virus (RSV) IN, 3’ OH recessed ODN, and strand transfer inhibitor (STI) used to assemble STI-trapped synaptic complex (SC); B: MK-2048 and Dolutegravir (DTG) efficiently produce trapped SC while Raltegravir had moderate efficiency. No trapped SC is produced in the absence of STI. Elution positions of STI-trapped SC (red line), free IN and DNA are marked. Molecular weight markers are indicated.
- Citation: Grandgenett DP, Pandey KK, Bera S, Aihara H. Multifunctional facets of retrovirus integrase. World J Biol Chem 2015; 6(3): 83-94
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/83.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.83