Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 218-222
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.218
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.218
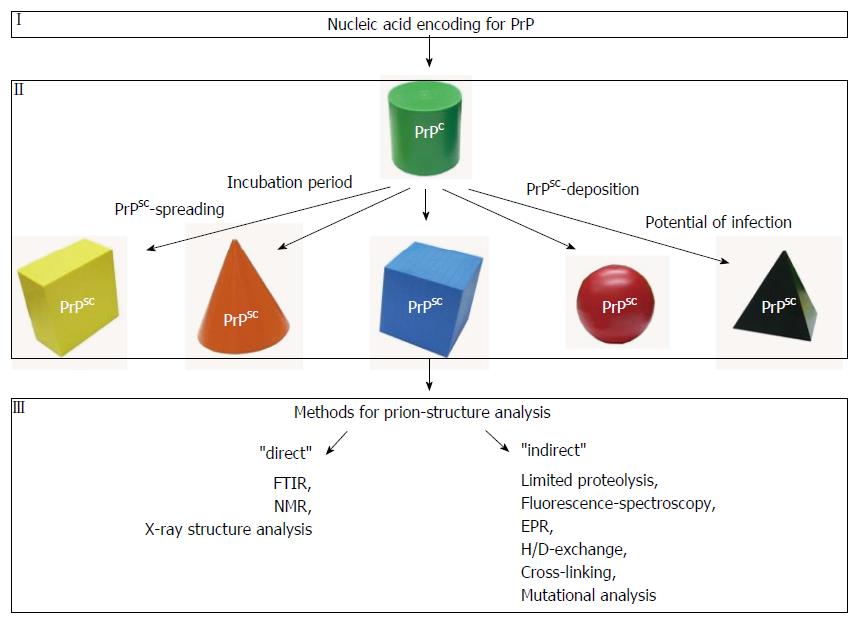
Figure 1 Different conformations of the prion protein.
In mammals and humans both isoforms of prion protein (PrP) [the cellular isoform (PrPC) and the misfolded isoform (PrPSC)] are encoded by the same gene (I). PrPC is shown in green (II). Under specific conditions PrPC can be transferred into a misfolded and then putatively infectious conformation (PrPSC). Prion strains can adopt different conformations (indicated by different symbols and colors). When a misfolded isoform of the prion protein becomes infectious it is characterized by specific features as incubation period, PrPSC-spreading, PrPSC-deposition and a distinct potential of infection. For structural analyses of misfolded prion proteins several methods are available. While FTIR, NMR and X-ray structure analyses provide information about the secondary structure as well as 3D-structural information (“direct”), limited proteolysis, fluorescence spectroscopy, EPR, H/D-exchange, cross-linking or mutational analyses reveal more “indirect” structural information that is focused on specific residues within the protein. FTIR: Fourier-transform infrared.
- Citation: Daus ML. Techniques to elucidate the conformation of prions. World J Biol Chem 2015; 6(3): 218-222
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/218.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.218