Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 110-120
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.110
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.110
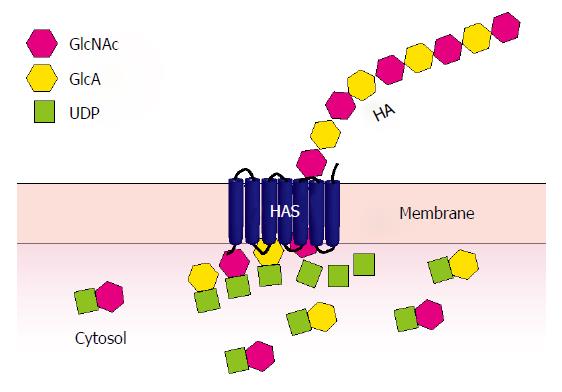
Figure 1 Hyaluronan synthesis.
Uridine diphosphate (UDP)-linked sugar substrates, glucuronic acid (GlcA) and N-acetylglucosamine (GlcNAc), are sequentially added to the reducing end of the growing HA chain at the cytoplasmic aspect of the cell membrane through the action of hyaluronan synthase (HAS). The resulting sugar polymer is extruded into the extracellular matrix surrounding the cell.
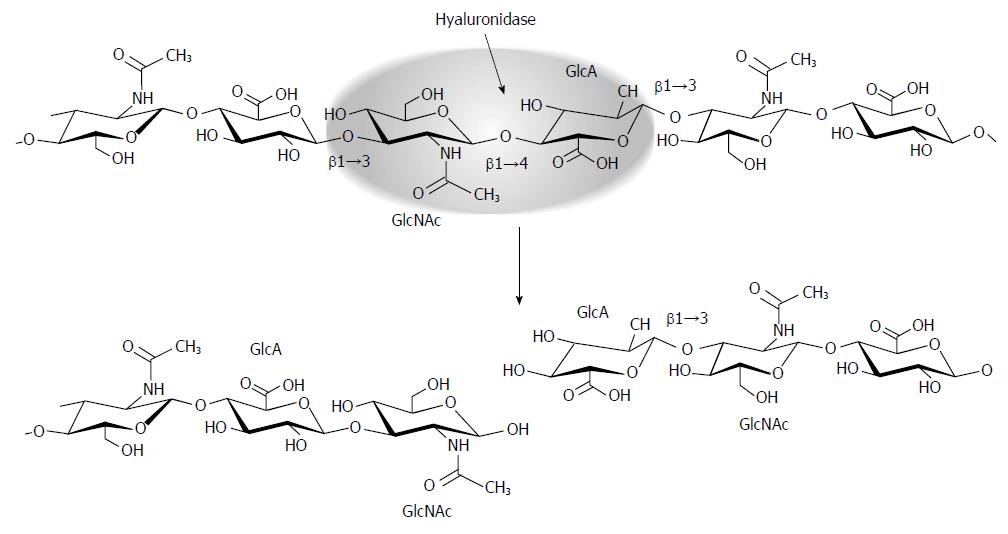
Figure 2 Hyaluronidase function.
Mammalian hyaluronidases are endoglycosidases that hydrolyze the β1→4 linkage between the GlcNAc and GlcA disaccharide units that make up HA. This results in non-reducing and reducing termini; the non-reducing sugar becomes a substrate for the exoglycosidase β-glucuronidase.
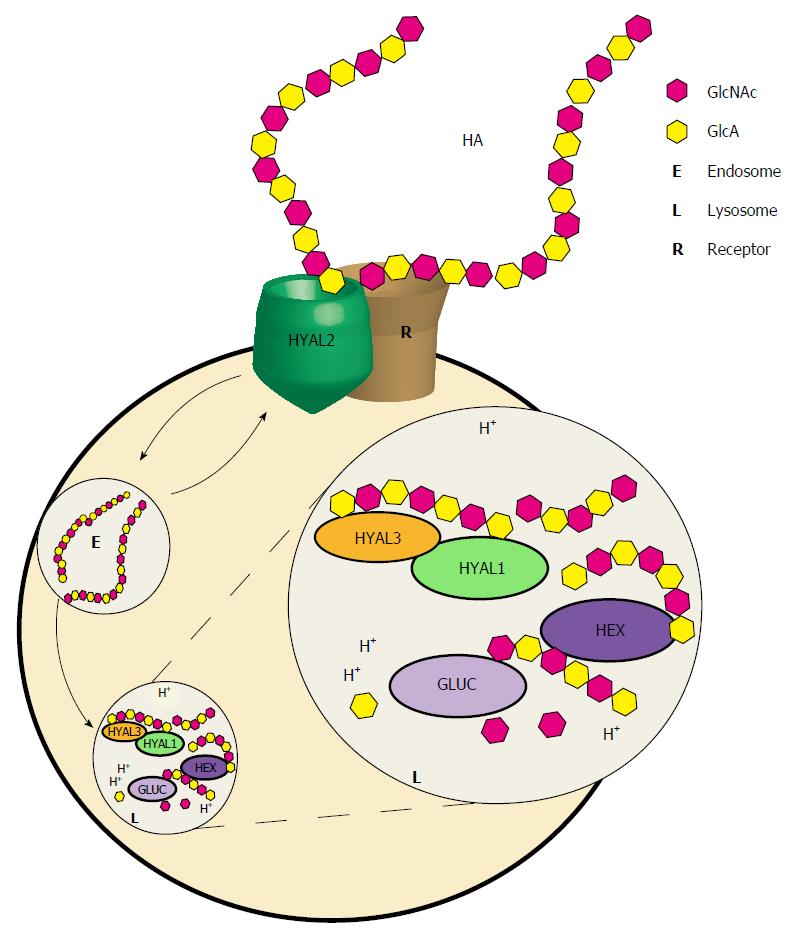
Figure 3 Proposed model for hyaluronan breakdown.
Hyaluronan (HA) bound by a cell- or matrix-associated receptor such as CD44, HARE, or LYVE-1 is proposed to be hydrolyzed to intermediate-sized fragments by the GPI-linked HYAL2. The resulting fragments are then internalized by receptor-mediated endocytosis and transported to lysosomes. Once inside the lysosome, further degradation takes place through the action of the acid-active HYAL1. HYAL1 cleaves the intermediate-sized HA fragments to smaller fragments such that they become substrates for the sequential action of the exoglycosidases, β-glucuronidase (Gluc) and β-N-acetylhexosaminidase (Hex) which hydrolyze terminal GlcA and GlcNAc, respectively. The role of HYAL3 is unclear although its overexpression increases HYAL1 activity in cell culture based studies.
- Citation: Triggs-Raine B, Natowicz MR. Biology of hyaluronan: Insights from genetic disorders of hyaluronan metabolism. World J Biol Chem 2015; 6(3): 110-120
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/110.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.110