Copyright
©2014 Baishideng Publishing Group Co.
World J Biol Chem. Feb 26, 2014; 5(1): 26-39
Published online Feb 26, 2014. doi: 10.4331/wjbc.v5.i1.26
Published online Feb 26, 2014. doi: 10.4331/wjbc.v5.i1.26
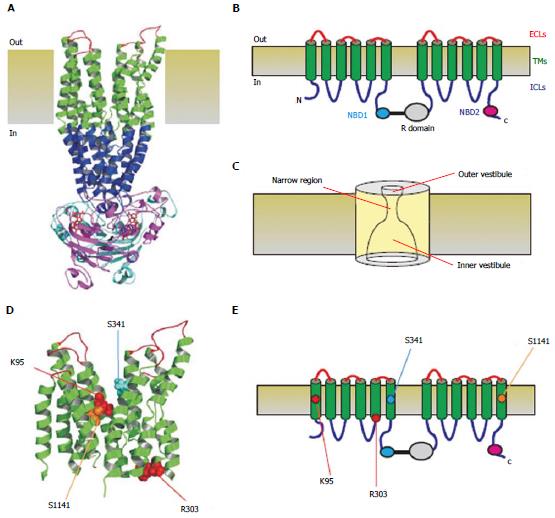
Figure 1 Three-dimensional and two-dimensional representations of cystic fibrosis transmembrane conductance regulator structure.
A: Atomic homology model of cystic fibrosis transmembrane conductance regulator in a so-called “channel like” conformation[20]. Different colours are used to illustrate the approximate extent of the extracellular loops (ECLs, red), transmembrane domains (TMs, green), intracellular loops (ICLs, blue), and two nucleotide binding domains (NBD1, cyan; NBD2, magenta). The cytoplasmic R domain is not included in this homology model; B: Schematic representation of these different domains (and the R domain), using the same colour scheme; C: Functional model of pore architecture. As described in the text, experimental evidence suggests that the pore has a narrow region that is connected to the cytoplasmic and extracellular solutions by a wide inner vestibule and a narrower outer vestibule, respectively; D: Location of putative blocker-interacting residues in the TMs (K95-TM1; R303-TM5; S341-TM6; S1141-TM12) within the same homology model shown in A. E: Location of these same residues in the same schematic model shown in B.
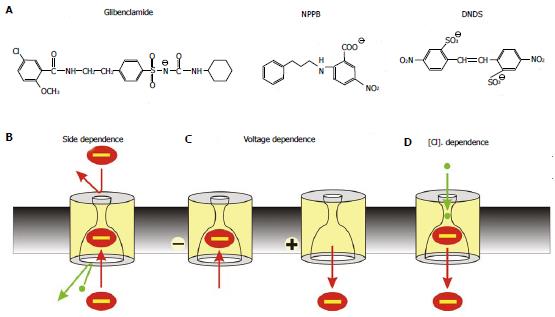
Figure 2 Mechanism of open channel blocker action.
A: Chemical structures of three well-known voltage-dependent cystic fibrosis transmembrane conductance regulator (CFTR) channel blockers: the sulfonylurea glibenclamide, the aryl amino benzoate 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), and the disulfonic stilbene 4,4’-dinitrostilbene-2,2’-disulfonic acid (DNDS). B-D: Characteristic functional properties of block shared by these and other CFTR open channel blockers: block is side-dependent, voltage-dependent, and sensitive to extracellular Cl- concentration; B: Blockers enter the pore only from its cytoplasmic end, likely because the extracellular entrance to the pore is too small and/or the narrow pore region prevents them from accessing their binding site in the wide inner vestibule; C: Block is relatively strengthened at hyperpolarized membrane potentials that favour entry of negative substances into the pore from the cytoplasm, and weakened at depolarized membrane potentials that favour anion retention in the cytoplasm; D: Block is weakened at higher extracellular Cl- concentrations; this is usually ascribed to a “knock-off” mechanism whereby Cl- entering the pore from its extracellular end electrostatically repels negatively charged blockers back into the cytoplasm, destabilizing blocker binding inside the pore.
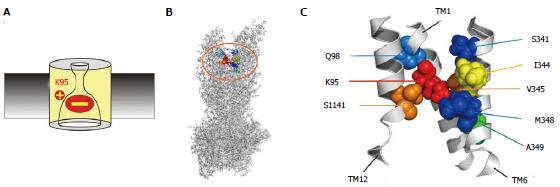
Figure 3 Location of amino acid residues key for blocker interactions in the pore inner vestibule.
A: The positively charged side chain of lysine residue K95 is essential for block, due to electrostatic attraction between this positive charge and the negatively charged blocker. However, this important charge can also be supported by other amino acid side chains that line the pore inner vestibule. B, C: Sites that have been shown to host positive charge that can support block are shown in an atomic homology model of the whole cystic fibrosis transmembrane conductance regulator protein (B) and in a detailed view of the central portions of TMs 1, 6 and 12 (C) the area highlighted in (B). The endogenous positively charged side chain of K95 is shown in red; those residues that were deemed best able to support this functionally important positive charge in orange (V345, S1141) or yellow (I344); and those that were able to host this positive charge to a lesser extent in blue (Q98, S341, M348) or green (A349). The homology model used here is the “channel like” conformation presented by ref[20] and shown in Figure 1A; other models give similar relative positions of these pore-lining side chains.
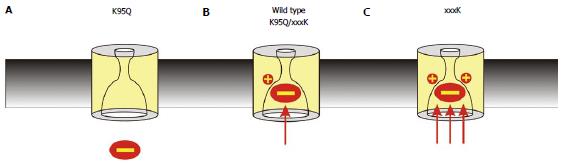
Figure 4 Importance of the number of fixed positive charges in the pore inner vestibule.
The importance of electrostatic interactions with the pore inner vestibule is demonstrated by the finding that the strength of block can be decreased or increased by mutations that decrease or increase, respectively, the number of positively charged amino acid side chains in the pore inner vestibule area shown in Figure 3C. A: Block is relatively weak in when the endogenous positive charge is removed, for example as in the K95Q mutation; B: Block is of similar strength to that observed in wild type cystic fibrosis transmembrane conductance regulator when the positive charge is “transplanted” to other, nearby sites, for example as in the double mutants K95Q/I344K, K95Q/V345K, and K95S/S1141K; C: Block is relatively strong when a second positive charge is introduced, for example as in I344K, V345K and S1141K.
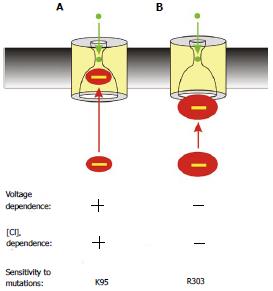
Figure 5 Two distinct proposed mechanisms of block by cytoplasmic anions.
A: The effects of most blockers are voltage- and Cl- dependent (as described in Figure 2) and are sensitive to mutations that remove the positive charge at K95; B: The large multivalent anion suramin blocks the channel in a voltage- and Cl- independent fashion, and its effects are dependent on a positive charge at R303 but independent of K95. This is interpreted as the large suramin molecule blocking the cytoplasmic entrance to the pore; at a site that does not involve entering significantly into the transmembrane electric field or approaching close enough to Cl- ions inside the pore to experience repulsive electrostatic interactions.
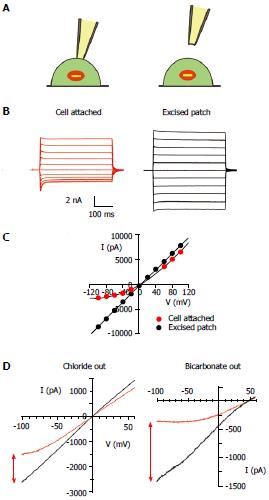
Figure 6 Channel block by cytoplasmic anions in intact cells and its dependence on extracellular anions.
A: During patch clamp recording from intact cells, cystic fibrosis transmembrane conductance regulator (CFTR) channels in the cell membrane are subject to block anions present in the cytoplasm of the cell (left). This blocking effect is lost when the patch of membrane is excised into the inside-out patch configuration (right); B: Example of this effect during macroscopic CFTR current recording from a baby hamster kidney cell expressing human CFTR, as described in detail[82]. Currents were recorded before (red) and after (black) excision of the patch from the cell, during voltage steps to between -100 mV and +100 mV in 20 mV increments from a holding potential of 0 mV. Dotted line represents the zero current level; C: Current-voltage relationships for the currents shown in (B). Note the outward rectification of the relationship in cell-attached recording (red) due to voltage-dependent channel block, and loss of this blocking effect following patch excision (black); D: Similar example current-voltage relationships from baby hamster kidney cell membrane patches when the extracellular solution contained 150 mmol/L NaCl (left) or 150 mmol/L NaHCO3- (right), as described in detail[101]. Note that the apparent degree of block in cell attached patches (red) is stronger when the extracellular solution contains HCO3- compared to Cl-, an effect quantified in detail in ref. [101].
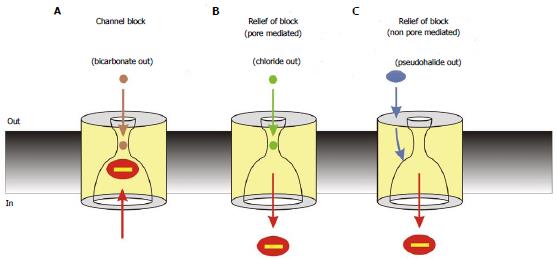
Figure 7 Interactions between cytoplasmic blocking anions and extracellular anions.
Cytoplasmic block is modified by extracellular anions by different mechanisms, leading to different degrees of block under different conditions. A: Block is strong in the absence of modulation of block by extracellular anions; physiologically, such a condition may occur during periods of epithelial HCO3- secretion, leading to strong block of cystic fibrosis transmembrane conductance regulator (CFTR) channel currents under these conditions[101]; B: Block is weakened by extracellular anions that can enter the channel pore, such as Cl-, due to an electrostatic “knock-off” mechanism. This may lead to increased CFTR channel currents during periods of epithelial Cl- secretion[101]; C: Block is weakened by extracellular anions that interact with an extracellular part of the protein involving extracellular loop 4. This is presumed to result in a long-range conformational change in CFTR that decreases the affinity of the cytoplasmic blocker binding site. This mechanism may allow pharmacological manipulation of CFTR activity by compounds that interact with the extracellular anion binding site[114]. Note that Cl- ions may also interact with intracellular blocking anions by the non-pore mediated effect shown in (C)[114].
- Citation: Linsdell P. Cystic fibrosis transmembrane conductance regulator chloride channel blockers: Pharmacological, biophysical and physiological relevance. World J Biol Chem 2014; 5(1): 26-39
- URL: https://www.wjgnet.com/1949-8454/full/v5/i1/26.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i1.26