Copyright
©2013 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2013; 4(2): 18-29
Published online May 26, 2013. doi: 10.4331/wjbc.v4.i2.18
Published online May 26, 2013. doi: 10.4331/wjbc.v4.i2.18
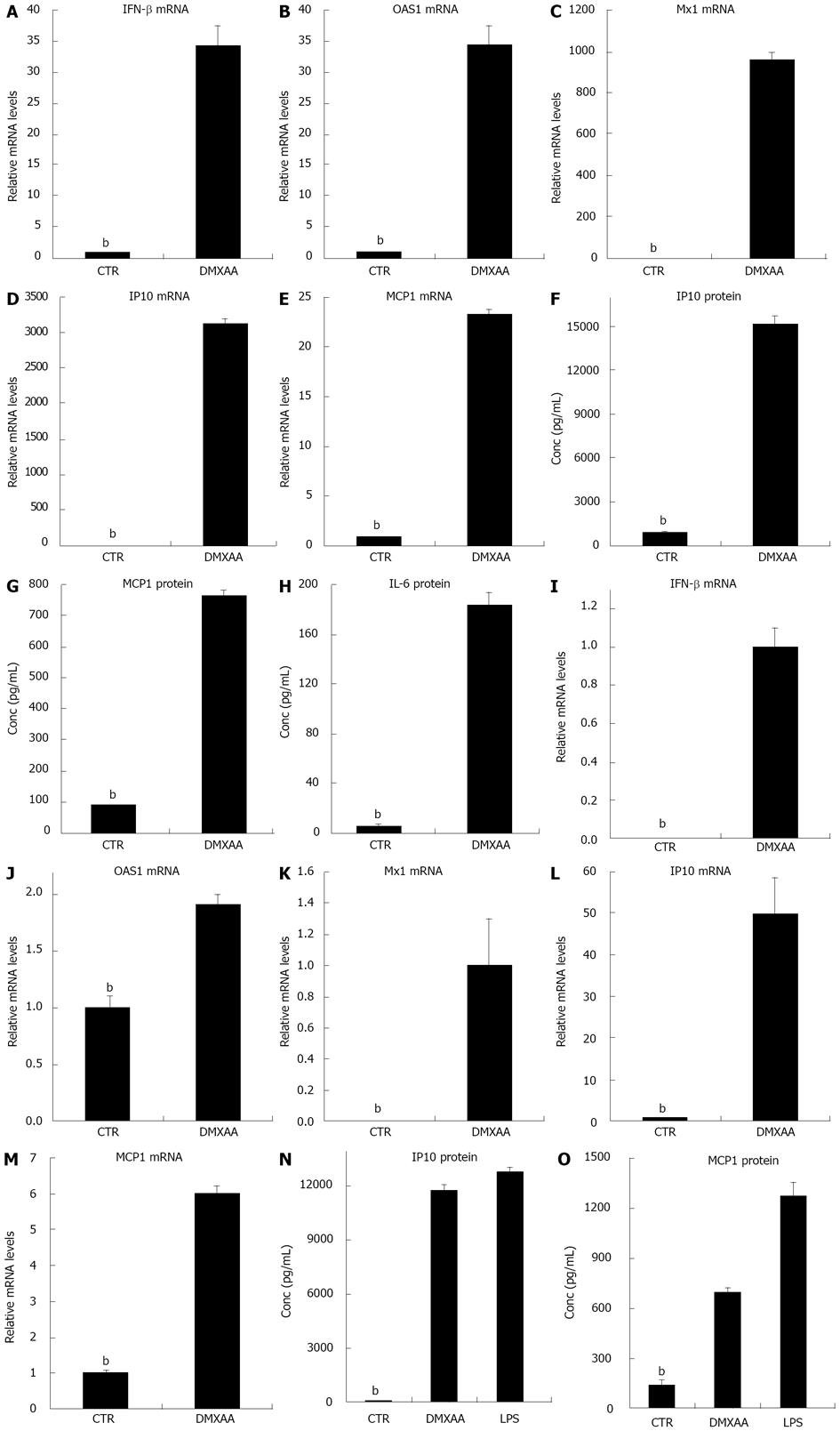
Figure 1 5,6-dimethylxanthenone-4-acetic acid elevates interferon-β-mediated antiviral genes and other chemokine/cytokine levels in mouse thioglycollate-elicited peritoneal and bone marrow derived macrophages.
A-E: Cells were incubated with or without 5,6-dimethylxanthenone-4-acetic acid (DMXAA) 100 μg/mL for 5 h. The total RNA was isolated and the relative interferon (IFN)-β, 2’,5’-oligoadenylate synthetase 1 (OAS1) and myxovirus resistance 1 (Mx1), inducible protein-10 (IP10), macrophage chemotactic protein (MCP1) and interleukin (IL)-6 mRNA levels were measured by real time reverse transcription-polymerase chain reaction; F-H: Induction of IP10, MCP1 and IL-6 protein was determined from the supernatant by enzyme-linked immunosorbent assay (ELISA); I-M: Cells were incubated with or without DMXAA 100 μg/mL or lipopolysaccharide (LPS) 100 ng/mL for 5 h. The total RNA was isolated and the relative IFN-β, OAS1 and Mx1, IP10, MCP1 and IL-6 mRNA levels were measured by real time reverse transcription-polymerase chain reaction; N,O: Induction of IP10 and MCP1 protein was determined from the supernatant by ELISA. The results presented as mean ± SE (bP < 0.01 vs DMXAA or LPS).
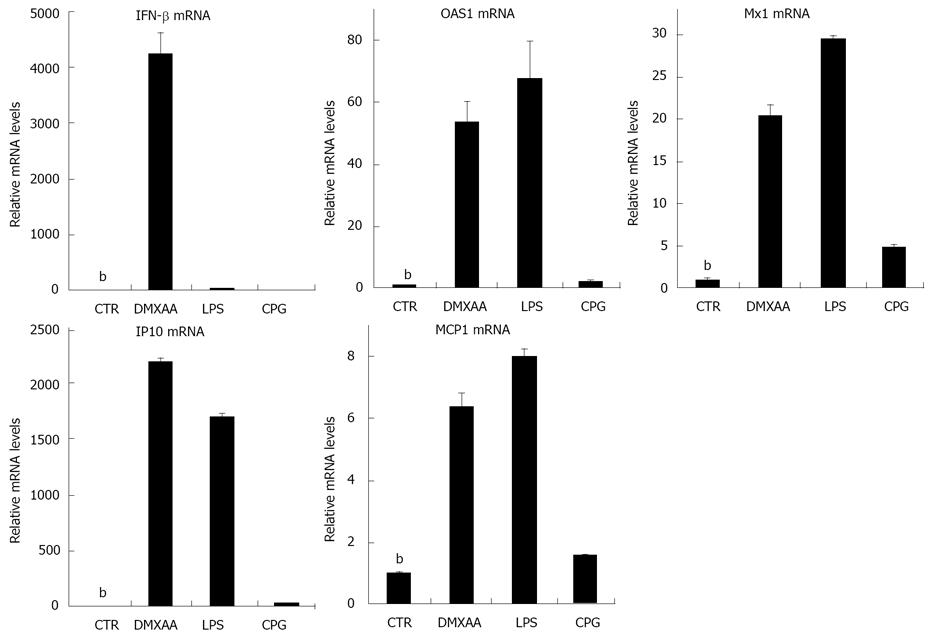
Figure 2 5,6-dimethylxanthenone-4-acetic acid activates interferon-β-mediated antiviral genes and other chemokine/cytokine levels in mouse bone marrow derived dendritic cells.
Cells were incubated with or without 5,6-dimethylxanthenone-4-acetic acid (DMXAA) 100 μg/mL, lipopolysaccharide (LPS) 100 ng/mL and CpG DNA 0.5 μmol/L for 6 h, respectively. The total RNA was isolated and the relative interferon (IFN)-β, 2’,5’-oligoadenylate synthetase 1 (OAS1) and myxovirus resistance 1 (Mx1), inducible protein-10 (IP10), macrophage chemotactic protein (MCP1) mRNA levels were measured by real time reverse transcription-polymerase chain reaction. The results presented as mean ± SE (bP < 0.01 vs DMXAA, LPS and CpG DNA, respectively).
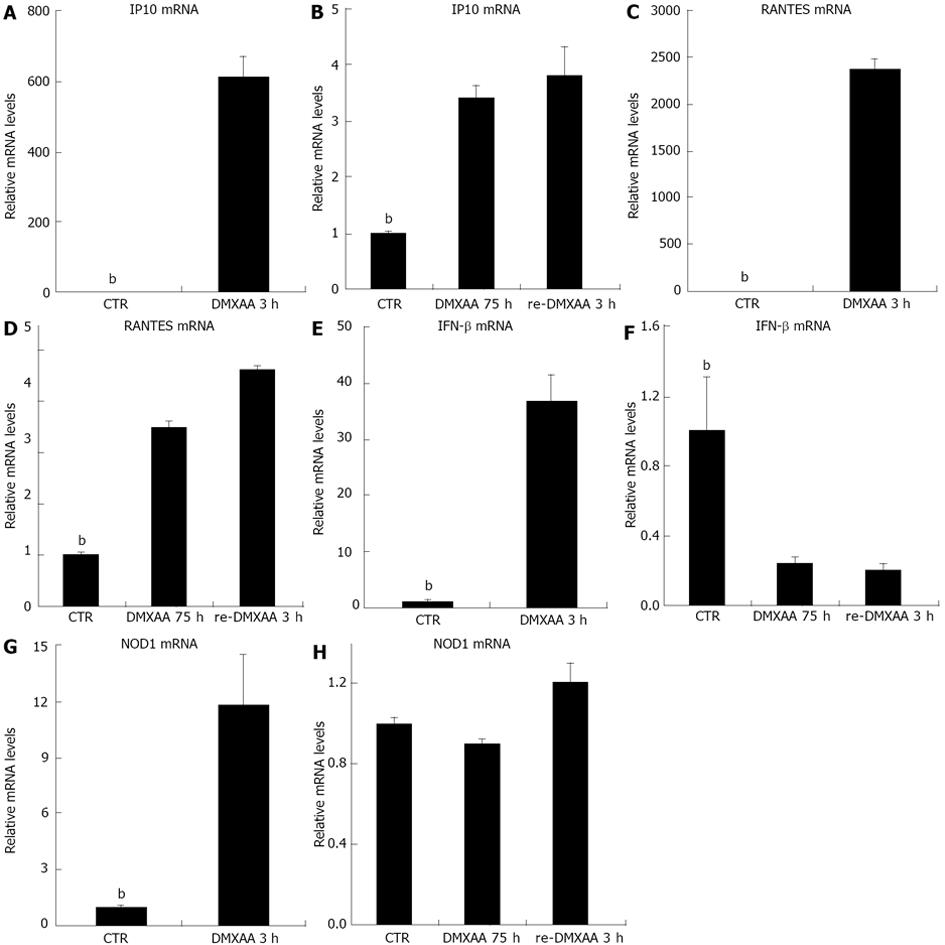
Figure 3 5,6-dimethylxanthenone-4-acetic acid pretreatment of C10 mouse bronchial epithelial cells induces refractoriness to secondary 5,6-dimethylxanthenone-4-acetic acid administration.
Cells were rechallenged with same concentration of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) 3 h after pretreatment with DMXAA 100 μg/mL for 72 h. The total RNA was isolated and the relative inducible protein-10 (IP10) (A, B), Rantes (C,D), interferon (IFN)-β (E, F) and nucleotide oligomerization domain 1 (NOD1) (G,H) mRNA levels were measured by real time reverse transcription-polymerase chain reaction. The results presented as mean ± SE (bP < 0.01 vs DMXAA groups).
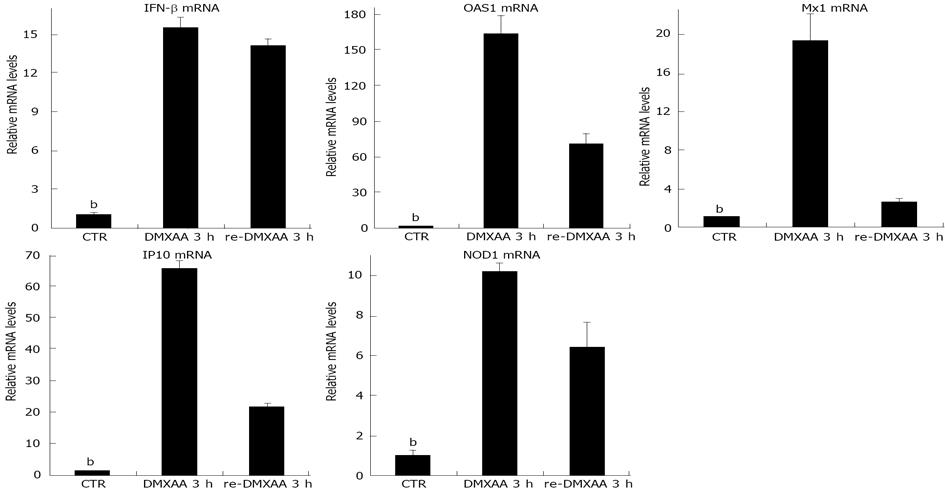
Figure 4 5,6-dimethylxanthenone-4-acetic acid induces refractoriness of interferon-β signaling in vivo.
Mice were rechallenged with 5,6-dimethylxanthenone-4-acetic acid (DMXAA) 3 h after pretreatment with DMXAA for 7 d, relative interferon (IFN)-β, 2’,5’-oligoadenylate synthetase 1 (OAS1) and myxovirus resistance 1 (Mx1), inducible protein-10 (IP10), and nucleotide oligomerization domain 1 (NOD1) mRNA levels of nasal epithelia were measured by real time reverse transcription-polymerase chain reaction. The results presented as mean ± SE (bP < 0.01 vs DMXAA group, 5 mice each group).
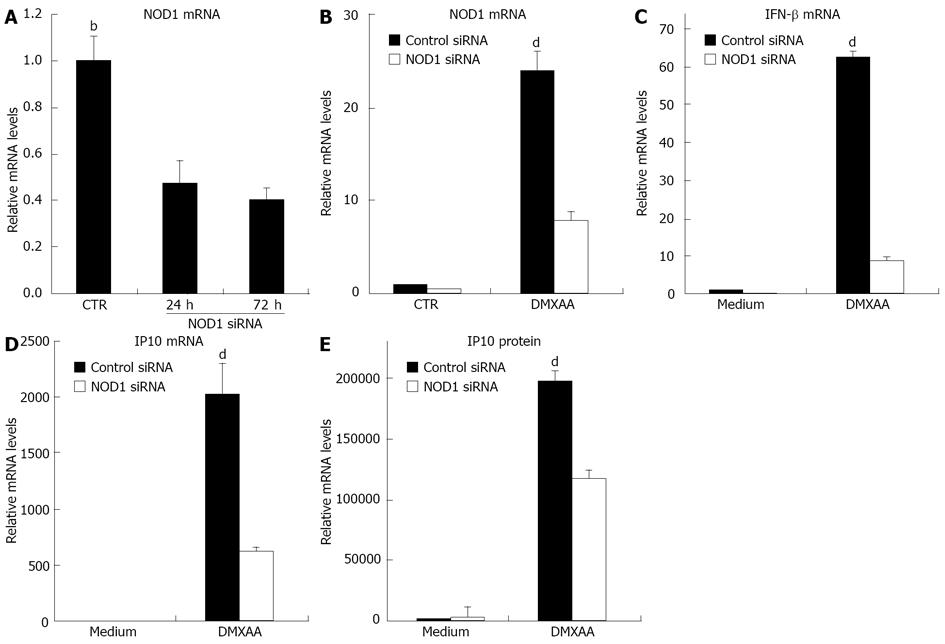
Figure 5 5,6-dimethylxanthenone-4-acetic acid-induced interferon-β expression inhibited by nucleotide oligomerization domain 1 small interfering RNA.
A: C10 cells were transfected with control small interfering RNA (siRNA) or nucleotide oligomerization domain 1 (NOD1) siRNA. Total RNA was extracted at indicated time points and the relative NOD1 mRNA levels were measured by real time real time reverse transcription-polymerase chain reaction (RT-PCR). After transfected with control siRNA or NOD1 siRNA for 72 h, C10 cells were further incubated with or without 5,6-dimethylxanthenone-4-acetic acid 100 μg/mL for 6 h; B-E: The relative NOD1, interferon (IFN)-β and inducible protein-10 (IP10) mRNA levels were measured by real time RT-PCR or the supernatants were removed and IP10 levels were measured by enzyme-linked immunosorbent assay (bP < 0.01 vs 24 h or 72 h; dP < 0.01 vs NOD1 siRNA, data are shown as the mean ± SE).
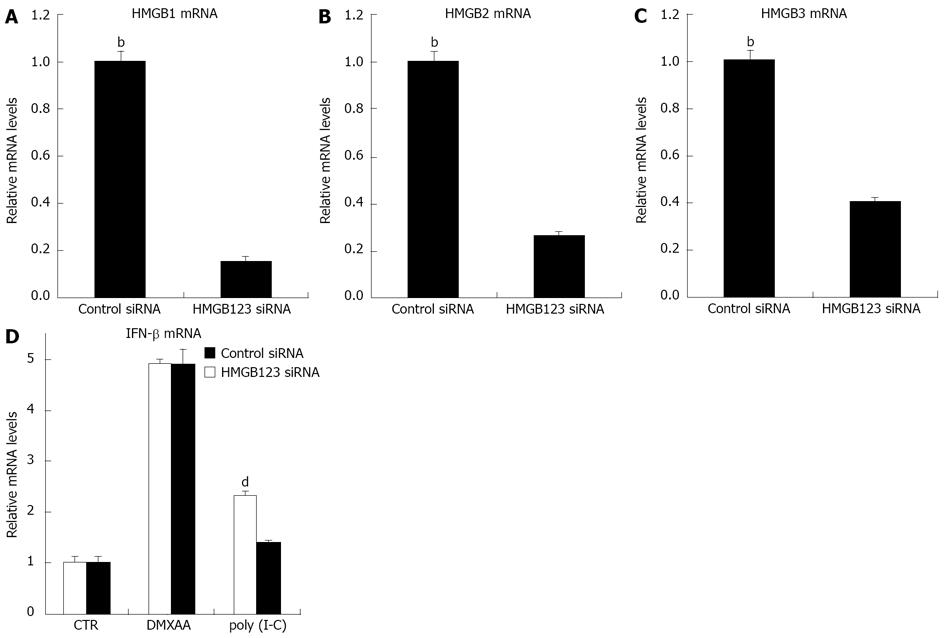
Figure 6 High-mobility group box 1/2/3 pathway did not involved in 5,6-dimethylxanthenone-4-acetic acid-induced interferon-β expression.
A-C: C10 cells were transfected with control small interfering RNA (siRNA) or high-mobility group box 1/2/3 (HMGB1/2/3) siRNA, the relative of each HMGB mRNA level was measured by real time reverse transcription-polymerase chain reaction (RT-PCR), respectively, HMGB1, HMGB2 and HMGB3; D: C10 cells were transfected with control siRNA or HMGB1/2/3 siRNA, then cells were further incubated with or without DMXAA or Poly I-C. The relative interferon (interferon) mRNA levels were measured by real time RT-PCR (bP < 0.01 vs HMGB1/2/3 siRNA; dP < 0.01 vs HMGB1/2/3 siRNA in Poly I-C group).
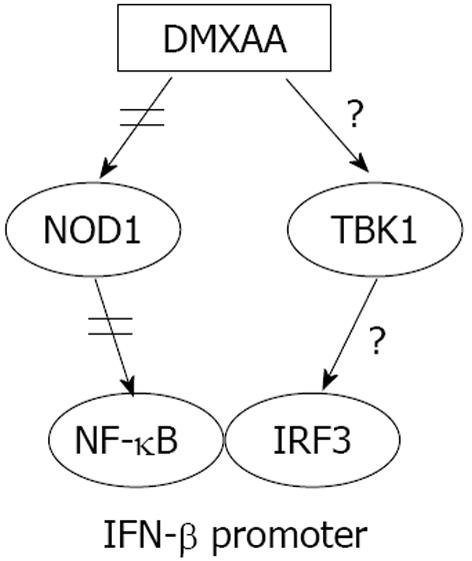
Figure 7 Schematic model of probable pathway of refractoriness of interferon-β signal.
Previous works have shown that 5,6-dimethylxanthenone-4-acetic acid (DMXAA) can activate TANK-binding kinase1 (TBK1) to initiate interferon (IFN) regulatory factor 3 (IRF3) signaling and nucleotide oligomerization domain 1 (NOD1) that interacts with rip-like interacting caspase-like apoptosis-regulatory protein kinase to activate nuclear factor-κB (NF-κB) signal pathway that regulates production of inflammatory cytokines/chemokines[11-13]. Our results indicate that DMXAA can induce refractoriness of interferon-β signal through NOD1 tolerance in mouse respiratory epithelial cells.
- Citation: Yu Z, Predina JD, Cheng G. Refractoriness of interferon-beta signaling through NOD1 pathway in mouse respiratory epithelial cells using the anticancer xanthone compound. World J Biol Chem 2013; 4(2): 18-29
- URL: https://www.wjgnet.com/1949-8454/full/v4/i2/18.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i2.18