Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. Jun 26, 2012; 3(6): 121-126
Published online Jun 26, 2012. doi: 10.4331/wjbc.v3.i6.121
Published online Jun 26, 2012. doi: 10.4331/wjbc.v3.i6.121
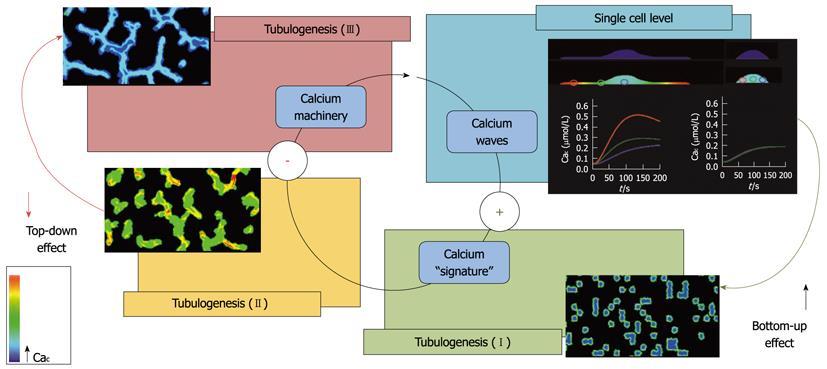
Figure 1 Scheme showing the relationship between calcium signaling and endothelial cell migration and organization in tubules in vitro (tubulogenesis).
Proangiogenic calcium signature triggers calcium-dependent machinery in single sparse endothelial cells (ECs). Calcium signature depends on the structural and functional state of any single cell (for the effect of cell shape, see 40: this event promotes migration during the early tubulogenic phases (tubulogenesis I and II; bottom-up effect). In mature tubules (tubulogenesis III) proangiogenic calcium machinery in ECs is inhibited (top-down effect).
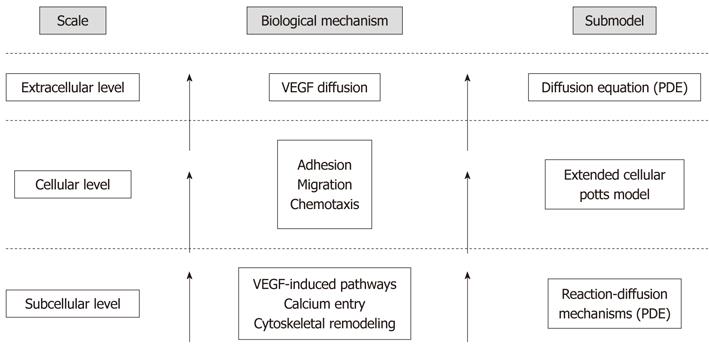
Figure 2 Hierarchy of scales and environments, with the corresponding biological mechanisms and modeling approaches, taken into account in the proposed model of tubulogenesis.
Our theoretical method spans the multiple levels involved in the process: it incorporates a continuous model of the specific agonist-induced, calcium-mediated intracellular cascades in a discrete cellular potts model, which represents the phenomenological evolution of the endothelial cell (EC) population. In particular, the microscopic dynamics alter the mesoscopic biophysical properties of the ECs, such as motility, compressibility, and chemotactic strength, affecting their behavior and, ultimately, the overall tubule formation. The different spatiotemporal levels, integrated in a hybrid framework, therefore directly affect each other and feedback over the whole simulation. VEGF: Vascular endothelial growth factor.
- Citation: Munaron L, Scianna M. Multilevel complexity of calcium signaling: Modeling angiogenesis. World J Biol Chem 2012; 3(6): 121-126
- URL: https://www.wjgnet.com/1949-8454/full/v3/i6/121.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i6.121