Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. Jun 26, 2012; 3(6): 110-120
Published online Jun 26, 2012. doi: 10.4331/wjbc.v3.i6.110
Published online Jun 26, 2012. doi: 10.4331/wjbc.v3.i6.110
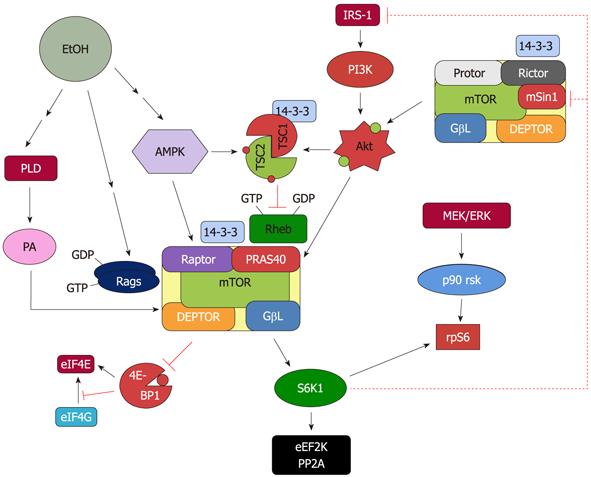
Figure 1 Proposed model for the regulation of mammalian target of rapamycin complex 1 and mammalian target of rapamycin complex 2 in response to EtOH.
EtOH decreases the activity of mammalian target of rapamycin complex (mTORC)1 toward its substrates 4E binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1). This process is mediated via multiple signaling routes including AMP-activated protein kinase (AMPK), phosphoinositol-3 kinase (PI3K)/Akt, phospholipase D (PLD) and Rag GTPases. The role each of these upstream regulators plays in affecting mTORC1 function is discussed in the text. The decrease in S6K1 phosphorylation with EtOH signals to mTORC2 as part of a feedback loop. As such, EtOH increases mTORC2 activity toward Akt, which then enhances phosphorylation of its substrate proline rich Akt substrate of 40 kDa (PRAS40).
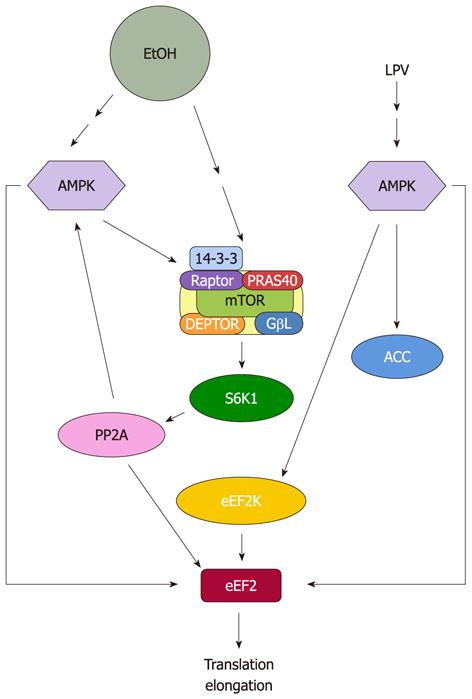
Figure 2 Proposed model for regulation of eukaryotic elongation factor 2 by EtOH and human immunodeficiency virus antiretroviral drug lopinavir.
EtOH and lopinavir (LPV) increase eukaryotic elongation factor 2 (eEF2) phosphorylation and inactivate this protein. This process is directly mediated by increased AMP-activated protein kinase (AMPK) activity. EtOH reduces eEF2 function owing to decreased protein phosphatase 2A (PP2A) activity, while LPV regulates this process by increasing eEF2 kinase (eEF2K) activity. mTOR: Mammalian target of rapamycin.
- Citation: Hong-Brown LQ, Kazi AA, Lang CH. Mechanisms mediating the effects of alcohol and HIV anti-retroviral agents on mTORC1, mTORC2 and protein synthesis in myocytes. World J Biol Chem 2012; 3(6): 110-120
- URL: https://www.wjgnet.com/1949-8454/full/v3/i6/110.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i6.110