Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2012; 3(5): 98-109
Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.98
Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.98
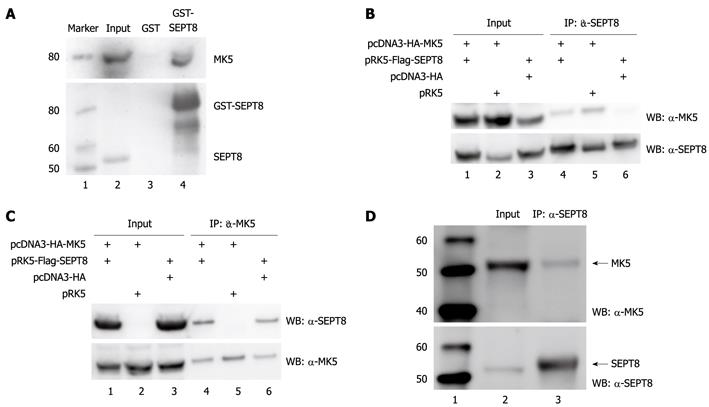
Figure 1 Mitogen-activated protein kinase-activated protein kinase 5 and SEPT8 interact in vitro and in vivo.
A: Glutathione S-transferase (GST) pull-down assay. GST-SEPT8 or GST alone purified from Escherichia coli and immobilized on glutathione-Sepharose beads were incubated for 60 min with lysate of HEK293 cells transfected with GFP-mitogen-activated protein kinase-activated protein kinase 5 (MK5). After washing the beads five times, bound proteins were eluted by boiling, subjected to SDS-PAGE, and immunoblotted with anti-PRAK (upper panel) or anti-SEPT8 (lower panel) antibody. Lane 1: Protein molecular mass marker (in kDa); lane 2: Cell lysate; lane 3: Cell lysate after pull down with GST; lane 4: Cell lysate after pull down with GST-SEPT8; B: Coimmunoprecipitation of FLAG-SEPT8 and hemagglutinin (HA)-MK5. HA-MK5- and/or FLAG-SEPT8-encoding plasmids were transiently expressed in HEK293 cells. Total cellular lysates (input; lanes 1-3) and SEPT8 immunoprecipitates (IP: α-SEPT8; lanes 4-6) were probed with anti-PRAK (upper panel) and anti-SEPT8 (lower panel) antibody. Lane 1: Cell lysate of cells cotransfected with expression plasmids for HA-tagged MK5 and FLAG-tagged SEPT8; lane 2: Cell lysate of cells cotransfected with expression plasmids for HA-tagged MK5 and empty vector for SEPT8; lane 3: Cell lysate of cells cotransfected with expression plasmids for FLAG-tagged SEPT8 and empty vector for MK5; lane 4: Immunoprecipitated lysate of cells cotransfected with expression plasmids for HA-tagged MK5 and FLAG-tagged SEPT8; lane 5: Immunoprecipitated lysate of cells cotransfected with expression plasmids for HA-tagged MK5 and empty vector for SEPT8; lane 6: Immunoprecipitated lysate of cells cotransfected with expression plasmids for FLAG-tagged SEPT8 and empty vector for MK5; C: Coimmunoprecipitation of HA-MK5 and FLAG-SEPT8. HEK293 cells were transiently transfected with expression plasmids for HA-MK5 and/or FLAG-SEPT8. Total cellular lysates (input) and HA-MK5 immunoprecipitates (IP: α-MK5) were probed with anti-SEPT8 (upper panel) and anti-PRAK (lower panel) antibody. Lanes 1-3: Lysates from transfected cells; lanes 4-6: Immunoprecipitation of cell lysates. See (B) for details; D: Coimmunoprecipitation of endogenous MK5 and SEPT8. Endogenous SEPT8 was immunoprecipitated from PC12 cells with anti-SEPT8 antibodies and the precipitate was analyzed for the presence of MK5 by anti-PRAK antibodies. Lane 1: Protein molecular mass marker (in kDa); lane 2: Lysate of PC12 cells (= input); lane 3: Immunoprecipitation with SEPT8 antibodies. The bottom panel shows control western blot with SEPT8 antibodies. IP: Immunoprecipitation; WB: Western blotting.
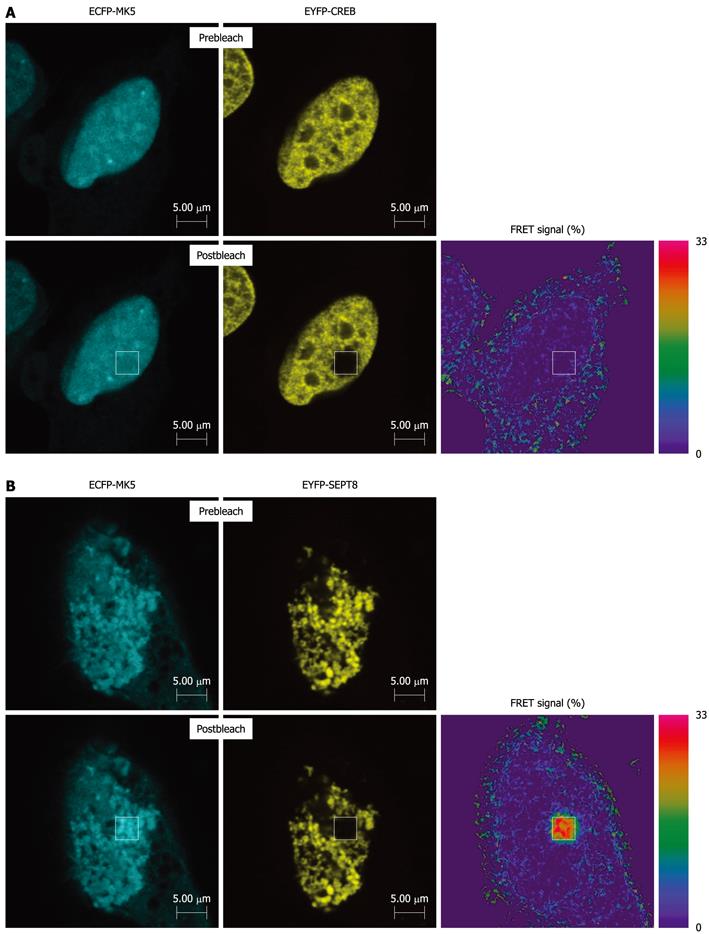
Figure 2 Fluorescence resonance energy transfer analysis shows interaction between mitogen-activated protein kinase-activated protein kinase 5 and SEPT8.
(A) HeLa cells were cotransfected with expression plasmids for heat shock protein (CFP)-tagged mitogen-activated protein kinase-activated protein kinase 5 (MK5) and yellow fluorescent protein (YFP)-tagged CREB or (B) with expression vectors for CFP-tagged MK5 and YFP-tagged SEPT8. Twenty-four hours after transfection, cells were fixed and fluorescence resonance energy transfer (FRET) analysis was carried out. CFP and YFP were excited with separate laser channels of 458 and 514 nm, respectively. Emission fluorescence intensity data were obtained at 465-500 nm (CFP) and 525-600 nm (YFP). CFP and YFP emission signals were captured before and after 50% photobleaching YFP. FRET is indicated as the relative increase in CFP emission following YFP photobleaching.
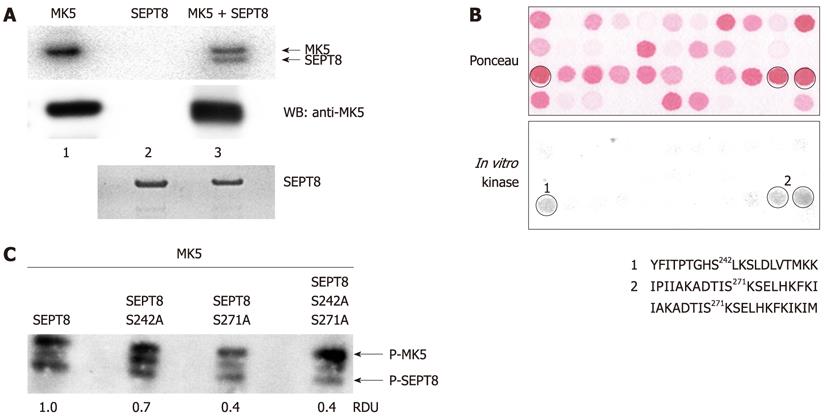
Figure 3 Mitogen-activated protein kinase-activated protein kinase 5 phosphorylates septin 8 in vitro.
A: In vitro kinase assay on recombinant SEPT8. Glutathione S-transferase (GST)-SEPT8 fusion protein was purified from Escherichia coli and the GST moiety was removed by thrombin. SEPT8 was incubated with activated mitogen-activated protein kinase-activated protein kinase 5 (MK5) for 30 min at 30 °C in the presence of [γ-32P] ATP. Proteins were separated by SDS-PAGE and phosphorylation was visualized by autoradiography (upper panel). Loading control for MK5 (middle panel) and SEPT8 (lower panel) was performed by western blotting with anti-PRAK and anti-SEPT8 antibodies, respectively. Lane 1: Recombinant activated MK5; lane 2: Purified SEPT8 protein; lane 3: recombinant MK5 and purified SEPT8; B: SEPT8 peptide array was subjected to in vitro phosphorylation by activated MK5. Each spot represents a 20-mer peptide fragment of SEPT8. The peptide fragments constitute the complete SEPT8 protein. The sequential peptide has a 17-amino acid overlap with the previous peptide. Several spots with peptides were detected by autoradiography (lower panel). Ser-242 and Ser-271 with highest prediction score 0.994 and 0.944, respectively by Netphos software, were chosen as possible phosphorylation sites in the peptide sequences. The upper panel represents Ponceau staining of the peptide array membrane used. The sequences of the peptides representing the positive spots are shown; C: In vitro phosphorylation of wild-type SEPT8, and SEPT8 mutants carrying a single amino acid substitution (Ser-242 into Ala or Ser-271 into Ala, respectively) or the double amino acid substitution Ser-242 and Ser-271 into Ala. The upper band represents autophosphorylated MK5, while the lower band is phosphorylated SEPT8. Relative densitometry units (RDU) of the bands representing phosphorylated SEPT8 are shown. The value obtain for wild-type SEPT8 was arbitrary set as 1.0 and the other values were related to this.
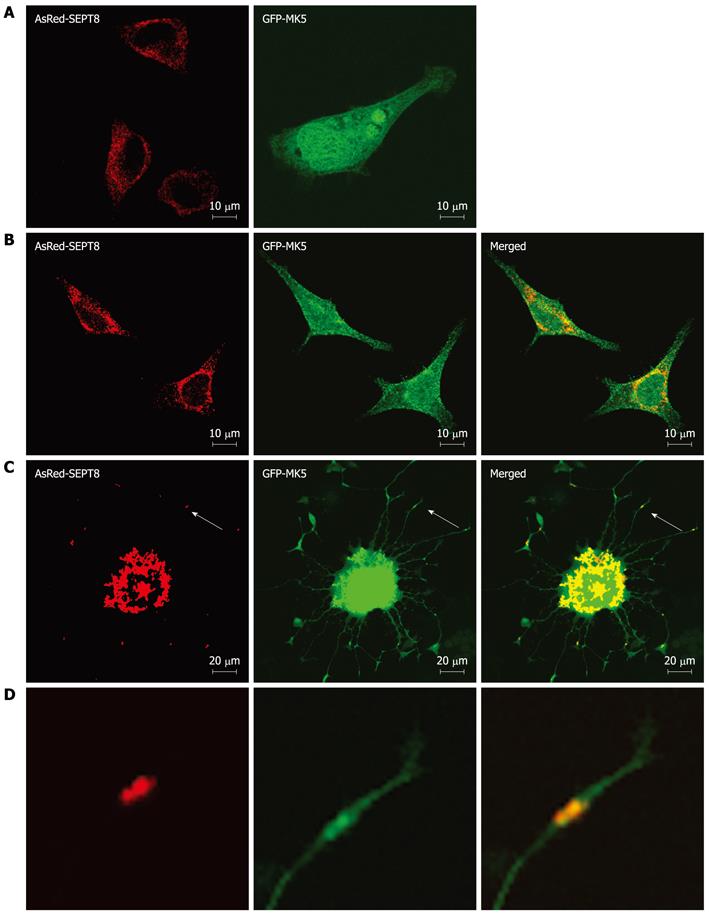
Figure 4 Mitogen-activated protein kinase-activated protein kinase 5 colocalizes with SEPT8.
A: HeLa cells were transfected separately with an expression plasmid for GFP-mitogen-activated protein kinase-activated protein kinase 5 (MK5) (left panel) or AsRed-SEPT8 (right panel); B: HeLa cells cotransfected with both expression plasmids. The subcellular localization of RFP-tagged SEPT8 (left panel), GFP-tagged MK5 (middle panel), or both proteins (merged; right panel) is shown; C: PC12 cells cotransfected with expression vectors for AsRed-SEPT8 (left panel) and GFP-MK5 (middle panel). Colocalization is shown in the right panel; D: Enlargement of the areas marked by arrows in figures (C) shows clear colocalization of MK5 and SEPT8. After 24 h, cells were fixed and MK5 (green channel) and SEPT8 (red channel) were visualized directly.
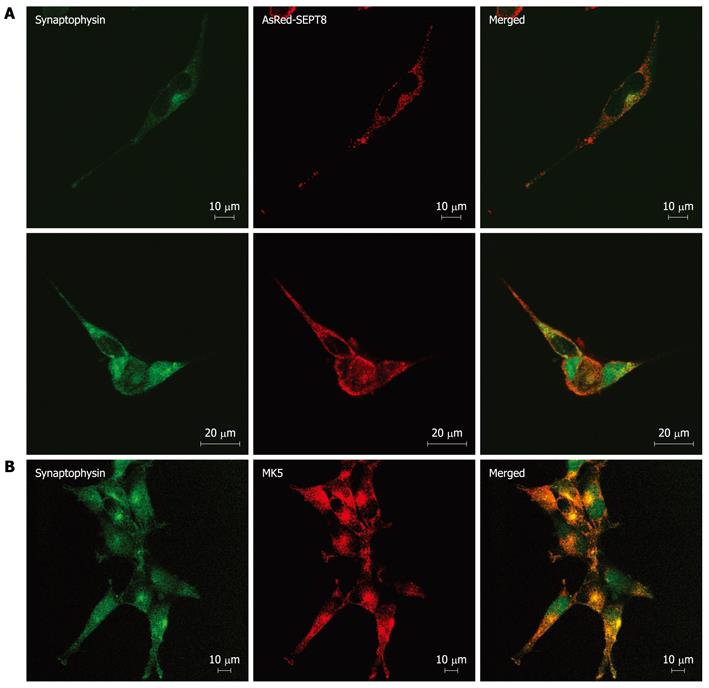
Figure 5 Mitogen-activated protein kinase-activated protein kinase 5 and SEPT8 colocalize with synaptophysin.
A: SK-N-DZ cells were transfected with expression vector encoding AsRed-SEPT8 and after 24 h, cells were fixed. Synaptophysin was visualized by staining with anti-synaptophysin fluorescein isothiocyanate (FITC) conjugate antibody (left panels), while AsRed-SEPT8 was visualized directly (red channel; middle panels). A merged image of red and green channels is shown in the right panel; B: SK-N-DZ cells were fixed and stained with anti-synaptophysin FITC conjugate antibody (left panel) and with anti-PRAK antibody followed by Alexa Fluor 568 anti-rabbit antibody (middle panel). Merged image of red and green channels is shown in the right panel.
Figure 6 Mitogen-activated protein kinase-activated protein kinase 5 phosphoacceptor sites in human SEPT8 are conserved in SEPT8 from other species.
Alignment of proven and putative SEPT8 proteins in different species of the region encompassing mitogen-activated protein kinase-activated protein kinase 5 phosphoacceptor sites Ser-242 and Ser-271 (numbering for human SEPT8). The code in parenthesis refers to the accession number in the Protein database (http://www.ncbi.nlm.nih.gov/protein/). Asterisk indicates identical amino acid residues. The non-conserved residues are shown in red. The one-letter amino acid code is used. The residues corresponding to human SEPT8 Ser-242 and Ser-271 are shown.
-
Citation: Shiryaev A, Kostenko S, Dumitriu G, Moens U. Septin 8 is an interaction partner and
in vitro substrate of MK5. World J Biol Chem 2012; 3(5): 98-109 - URL: https://www.wjgnet.com/1949-8454/full/v3/i5/98.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i5.98