Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. Jan 26, 2012; 3(1): 7-26
Published online Jan 26, 2012. doi: 10.4331/wjbc.v3.i1.7
Published online Jan 26, 2012. doi: 10.4331/wjbc.v3.i1.7
Figure 1 Schematic representation of rRNA processing pathway in Saccharomyces cerevisiae.
The full-length rRNA precursor undergoes several cleavage steps to produce functional 18S, 5.8S and 25S rRNAs for 60S and 40S ribosomes. Green trapezoids indicate points in the processing pathway at which Mtr4p activity has been shown to play a role. Also indicated are steps of processing that have been found to be completed in the cytoplasm[7,11].
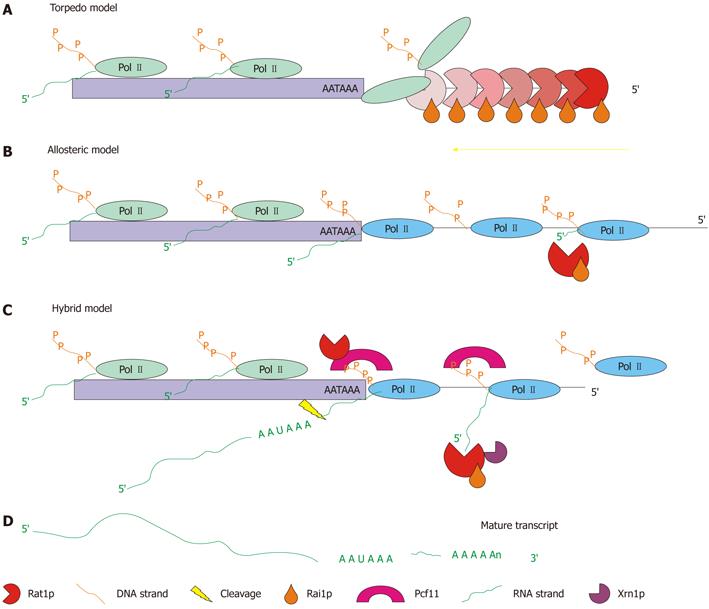
Figure 2 Schematic representation of the three possible modes of operation for the 5′→3′ exonuclease pathway.
A: Torpedo model-the Rat1p/Rai1p complex acts as a wedge to torpedo the polymerase from the DNA strand and terminate transcription[53,54]; B: Allosteric model-the positioning element for the poly(A) site (AATAAA) causes a change in the conformation of the polymerase causing a gradual stop in transcription. The Rat1p/Rai1p complex is used to eliminate RNA downstream of the poly(A) site[55]; C: Hybrid model-the positioning element for the poly(A) site causes a change in the conformation of the polymerase, while Rat1p and Pcf11 also cause a pause in transcription. Termination is gradual but faster than that in the allosteric model. Rat1p/Rai1p are still utilized to remove RNA transcribed downstream of the poly(A) site (adapted from[56]); D: The end result of each model is a mature transcript.
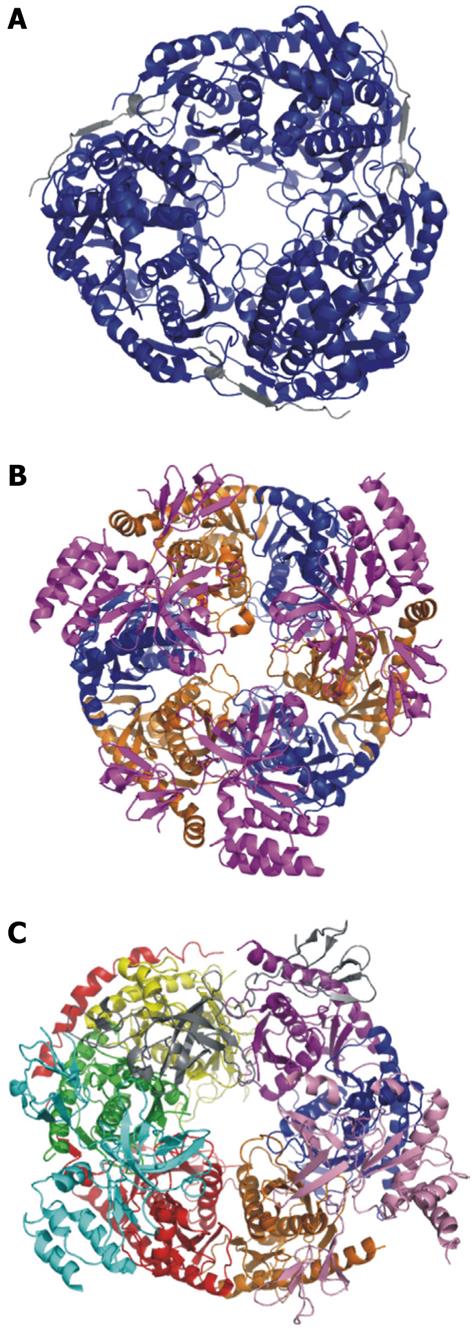
Figure 3 Crystal structures of the bacterial PNPase, archaeal exosome, and human exosome.
A: Escherichia coli PNPase. Pictured in blue is the polynucleotide nucleotidyltransferase domain. In grey is the associated RNAse E domain (RCSB # 3GCM). The S1 and KH domains are not pictured because they are not included in the crystallized complex; B: Archaeal exosome. In blue is aRrp42, in orange is aRrp41. These two proteins are the exonucleolytic core proteins. In magenta is the aRrp4 cap protein (RCSBID: 2BA0); C: Human exosome. In orange is Rrp41p, in Blue is Rrp42p, in yellow is Rrp43p, in red is Rrp45p, in green is Rrp46p, in purple is Mtr3p. These six proteins make up the scaffolding ring structure. Also pictured are the cap proteins; in pink is Rrp4, in cyan is Rrp40, and in grey is Csl4 (RCSBID: 2NN6). The S. cervisiae exosome has not been crystalized but the human and yeast exosomes have approximately 51% sequence identity[51,66,143].
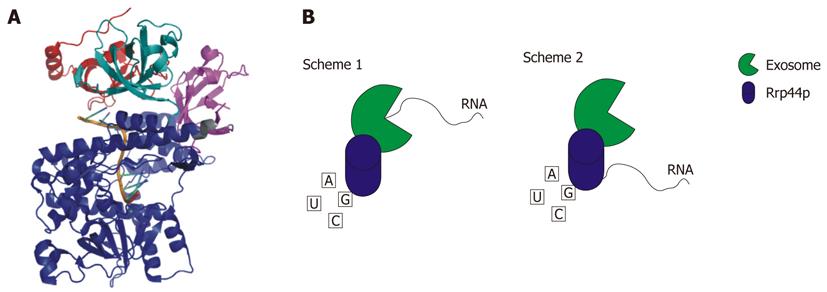
Figure 4 Rrp44p.
A: Crystal structure of Saccharomyces cerevisiae Rrp44p bound to RNA. CSD1 is colored red, CSD2 is colored teal, the RNB domain is blue, and the S1 domain is magenta. The PIN domain is not pictured because this domain is not included in the crystallized construct (RCSB # 2VNU); B: Schematic representation of the two mechanisms whereby Rrp44p is able to degrade RNA. In Scheme 1 the RNA is fed through the exosome to Rrp44p. Scheme 2 shows the RNA being degraded directly by Rrp44p[4,144].
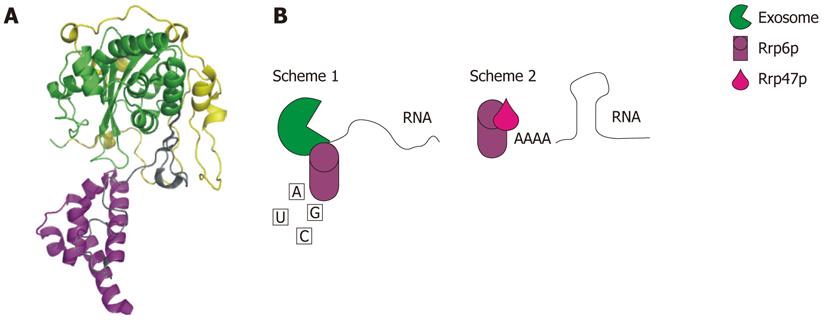
Figure 5 Rrp6p.
A: Crystal structure of Saccharomyces cerevisiae Rrp6p. The NTD is colored yellow, the exonuclease domain is colored green, and the HRDC domain is colored purple (RCSBID: 2HBJ); B: Schematic representation of the two different ways that Rrp6p functions in RNA degradation and processing. Scheme 1: Rrp6p interacts with the nuclear exosome to facilitate processing or degradation; Scheme 2: Rrp6p interacts with Rrp47 allowing Rrp6p to bind to RNA with secondary structure[145].
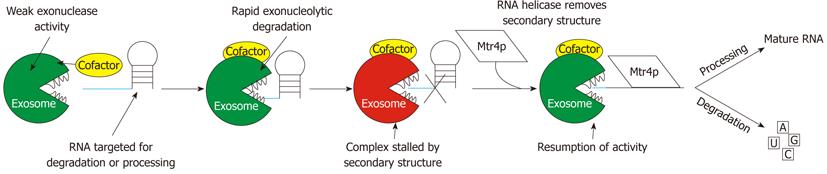
Figure 6 Schematic representation of the basic mode of operation of the 3′→5′ RNA exosome.
The figure highlights the need for a cofactor to stimulate the exonuclease activity of the exosome and the need for an RNA helicase to remove secondary structure to allow proper processing or degradation. The figure shows a representative stem loop structured RNA but any RNA with secondary structure could undergo the same remodeling to complete processing or degradation. The cofactor shown is a representative of all known and unknown cofactors. The exosome shows weak exonuclease activity in vitro, yet rapid degradation is seen in vivo, indicating that cofactors are required for this activity[76,80].
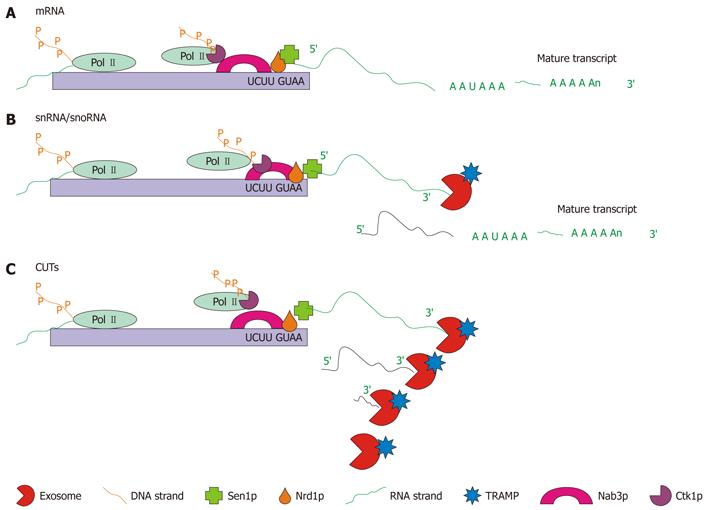
Figure 7 Schematic representation of the three types of Nrd1p/Nab3p-dependent RNA maturation.
A: mRNA 3′ end trimming: for some mRNAs, the Nrd1p complex is required to complete transcription. Pictured is the Nrd1p complex associated with Pol II bound to the Nrd1p and Nab3p binding sites, which cause the Pol II molecule to lift off of the DNA and release the RNA. At this point a poly(A) polymerase (not shown) polyadenylates the end of the strand producing the mature mRNA; B: snRNA/snoRNA 3′ end trimming: snRNA and snoRNA, which are transcribed from autonomous transcription units, are terminated by the Nrd1p complex. Pictured is the Nrd1p complex which causes transcription termination. Following polyadenylation by the TRAMP complex and 3′ end trimming by the exosome, the mature transcript is formed; C: Cryptic unstable transcript (CUT) degradation: transcription of CUTs is also terminated by the Nrd1p complex. Pictured is the Nrd1p complex interacting with Pol II causing transcription termination. Following termination the transcript is degraded by the exosome in conjunction with the TRAMP complex[86,95].
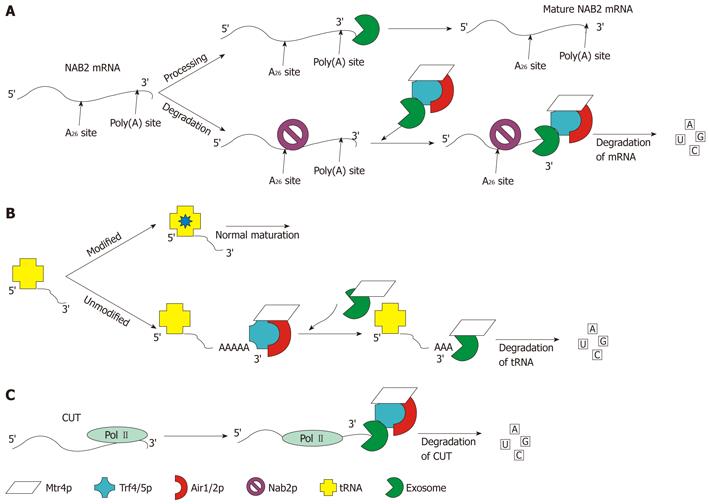
Figure 8 Schematic representation of the three well-characterized functions of the TRAMP4/5 complex.
A: Processing and degradation of NAB2 mRNA: the level of NAB2 mRNA is controlled by Nab2p, which recruits TRAMP and the exosome by binding to the A26 site of the mRNA. Once TRAMP and the exosome are recruited the transcript is degraded; B: Degradation of un-modified/misfolded tRNA: tRNA that has not undergone modification at the correct time is preferentially polyadenylated by TRAMP. Once polyadenylated, the exosome aided by Mtr4p degrades the tRNA; C: ncRNA degradation: shown in panel C is the degradation of CUTs. The same pathway is followed for snRNA and snoRNA, which are processed and degraded by TRAMP and the exosome. Degradation of CUTs is performed co-transcriptionally as pictured. The levels of these RNAs cannot be detected without depletion of Rrp6p or Trf4p[92,111,112].
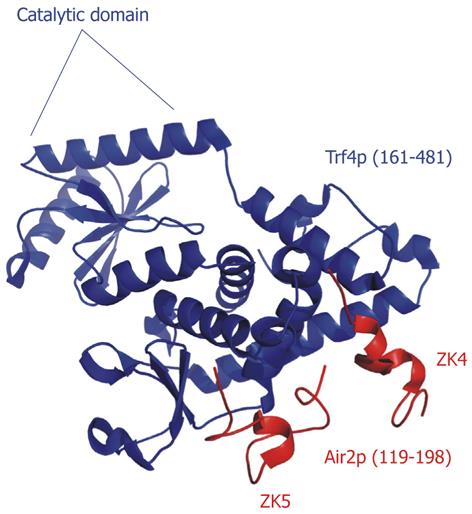
Figure 9 Crystal structure of the TRAMP4 core.
The catalytic domain of Trf4p is indicated. The fourth and fifth zinc knuckles (ZK4 and ZK5) pack against the central domain of Trf4p (RCSBID: 3NYB)[113].
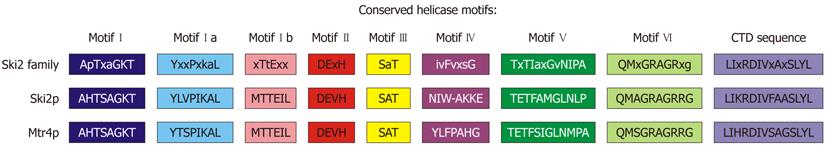
Figure 10 Ski2 family helicase motifs.
A multiple sequence alignment of the Ski2 family conserved helicase motifs and the C-terminal domain terminal sequence. Shown are alignments of the Ski2 family of helicases with the cytoplasmic Ski2p Saccharomyces cerevisiae helicase and Mtr4p. In each of the pictured regions, the two related helicases are highly conserved.
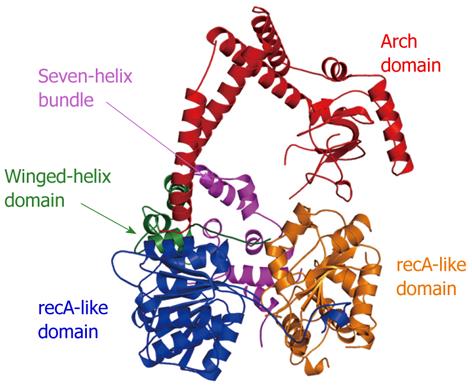
Figure 11 Crystal structure of apo-Mtr4p.
The individual domains (recA-like, winged-helix, seven-helix bundle, and arch) are labeled and colored similarly to the scheme in[138] (RCSBID: 3L9O). The arch domain, despite being in an ideal position to promote protein interactions, does not appear to interact with the TRAMP complex[140].
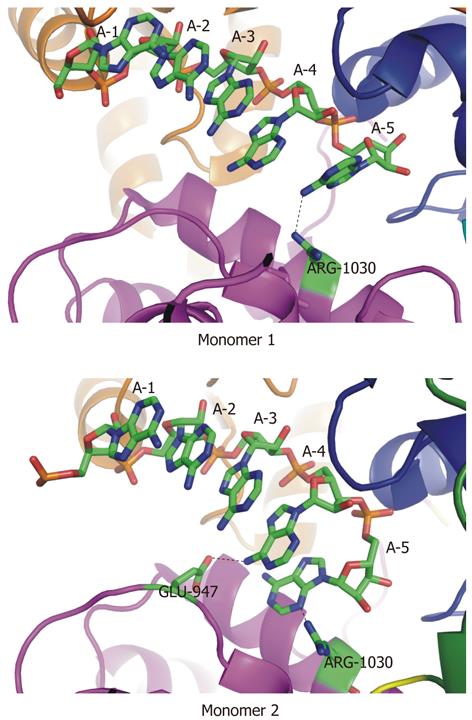
Figure 12 Two unique sets of contacts between Mtr4p and a short poly(A) substrate.
In two independent monomers in the asymmetric unit of the crystal structure (RCSBID: 2XGJ), two different sets of interactions with adenine bases at the 3′-end of the bound RNA are observed. A: In monomer 1, R1030 interacts with the exocyclic amino group of A5; B: In monomer 2, E947 interacts with the exocyclic amino group of A4 and R1030 interacts with the N3 nitrogen of A5.
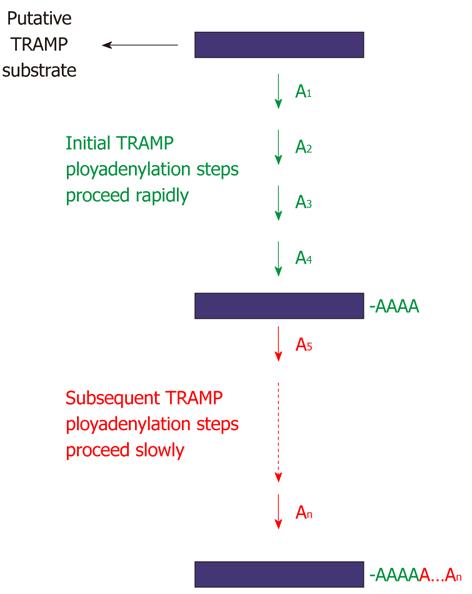
Figure 13 Regulation of TRAMP4 polyadenylation by Mtr4p.
Mtr4p has a novel function as a regulator of TRAMP polyadenylation. Addition of the first four adenylates to a TRAMP substrate is accelerated by Mtr4p, whereas the subsequent adenylates (i.e., A5 to An) are added more slowly, suggesting that interrogation of the 3′-end of a substrate by Mtr4p plays a significant role in this regulation[139].
- Citation: Bernstein J, Toth EA. Yeast nuclear RNA processing. World J Biol Chem 2012; 3(1): 7-26
- URL: https://www.wjgnet.com/1949-8454/full/v3/i1/7.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i1.7