Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Sep 26, 2011; 2(9): 202-214
Published online Sep 26, 2011. doi: 10.4331/wjbc.v2.i9.202
Published online Sep 26, 2011. doi: 10.4331/wjbc.v2.i9.202
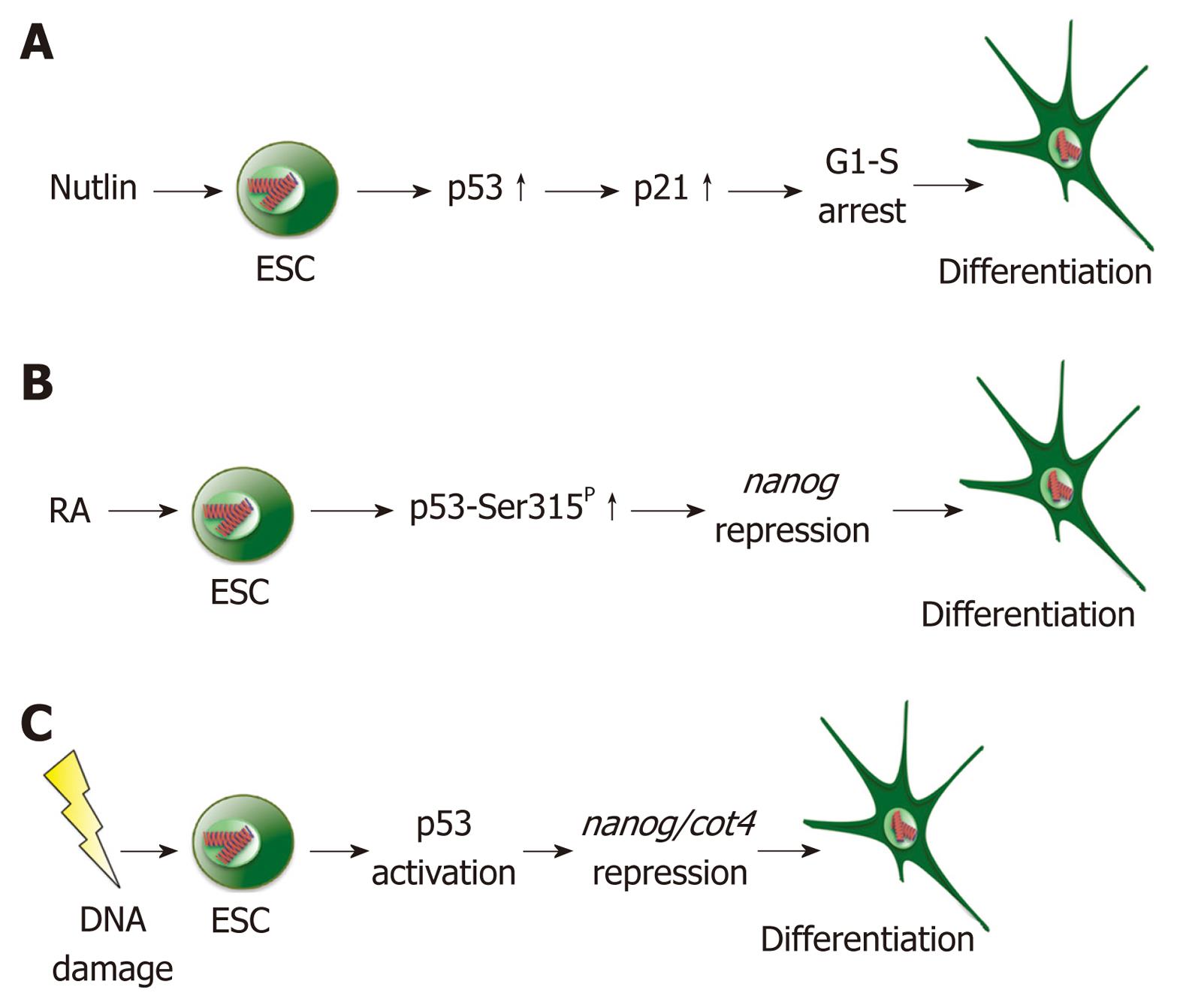
Figure 1 Differentiation of embryonic stem cells by p53.
A: Treatment of embryonic stem cells (ESCs) with nutlin leads to p53 accumulation and transcriptional activation. Activated p53 stimulates transcription of p21, whose gene product initiates cell cycle arrest at the G1/S border and differentiation; B: Treatment of ESCs with retinoic acid (RA) leads to phosphorylation of p53 at serine 315 and repression of nanog followed by differentiation; C: Irradiation of ESCs with UV light or treatment with doxorubicin leads to activation of p53. Activated p53 represses transcription of nanog and oct4, resulting in the differentiation of ESCs.
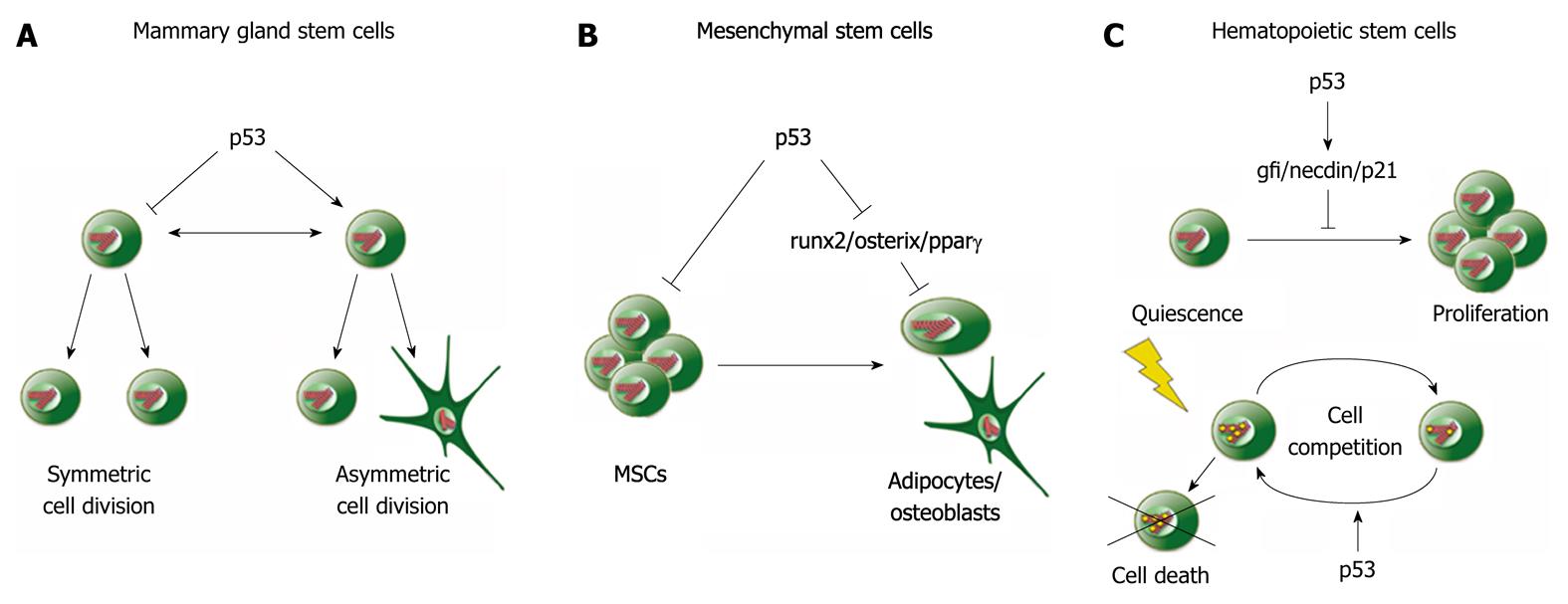
Figure 2 p53 suppresses self-renewal and promotes differentiation of adult stem cells.
A: In mammary gland stem cells, p53 promotes asymmetric cell division, resulting in an increased number of differentiated cells and a reduction in the stem cell pool; B: In mesenchymal stem cells (MSCs), p53 suppresses proliferation and self-renewal and reduces differentiation by suppressing runx2, osterix and pparγ; C: p53 supports quiescence of hematopoietic stem cells and reduces proliferation. This effect is mediated by transcriptional activation of gf1, necdin and p21 (Top). In response to DNA damage, p53 enhances the death of severely damaged cells by cell competition (below).
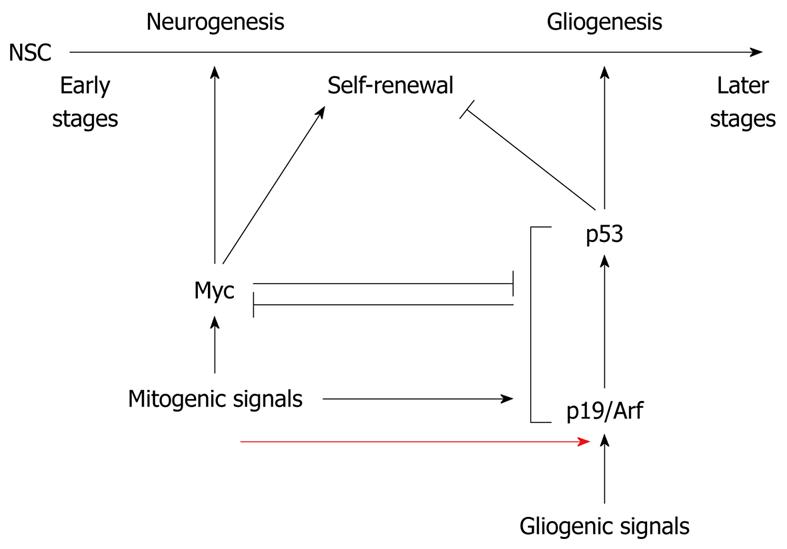
Figure 3 Antagonistic regulation of differentiation of neural stem cells by Arf/p53 and Myc.
Myc regulates self-renewal of neural stem cells (NSCs) and enhances their differentiation into the neuronal lineage. At early stages of embryogenesis, myc expression is high and p19Arf expression is low. Later in development, mitogenic signals upregulate p19Arf expression, resulting in enhanced abundance of the p53 tumor suppressor protein, which suppresses self-renewal of NSCs and differentiation towards the neuronal lineage while it supports gliogenesis [100].
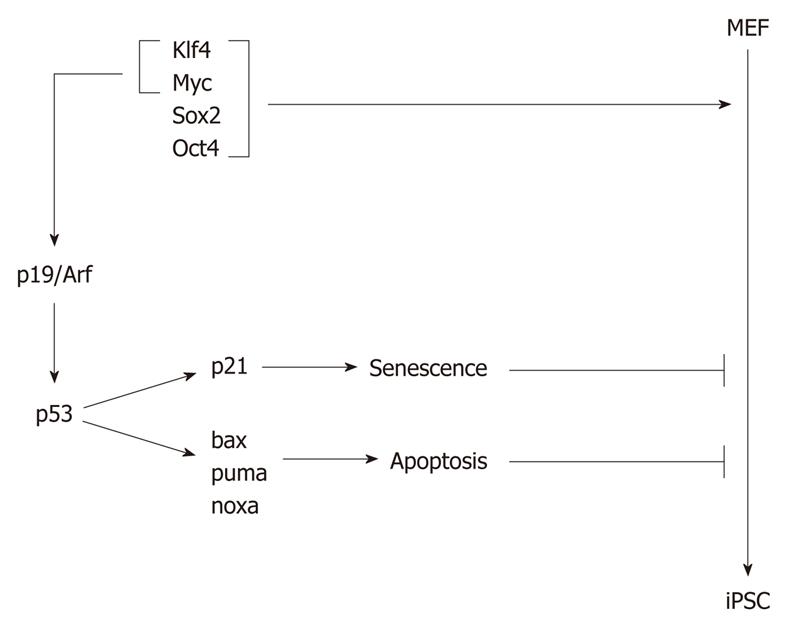
Figure 4 p53 provides a barrier for the reprogramming of somatic cells to induced pluripotent stem cells.
Somatic cells, such as mouse embryonic fibroblasts (MEFs), can be reprogrammed to induced pluripotent stem cells (iPSCs) after overexpression of a combination of reprogramming factors, such as Klf4, Sox2, Myc and Oct4. Klf4 and Myc induce p19Arf expression, which prevents p53 degradation, resulting in its accumulation and expression of its target genes. Among the target genes of p53 is p21, which causes senescence, and bax, puma and noxa, which lead to apoptosis.
- Citation: Solozobova V, Blattner C. p53 in stem cells. World J Biol Chem 2011; 2(9): 202-214
- URL: https://www.wjgnet.com/1949-8454/full/v2/i9/202.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i9.202