Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Jun 26, 2011; 2(6): 146-160
Published online Jun 26, 2011. doi: 10.4331/wjbc.v2.i6.146
Published online Jun 26, 2011. doi: 10.4331/wjbc.v2.i6.146
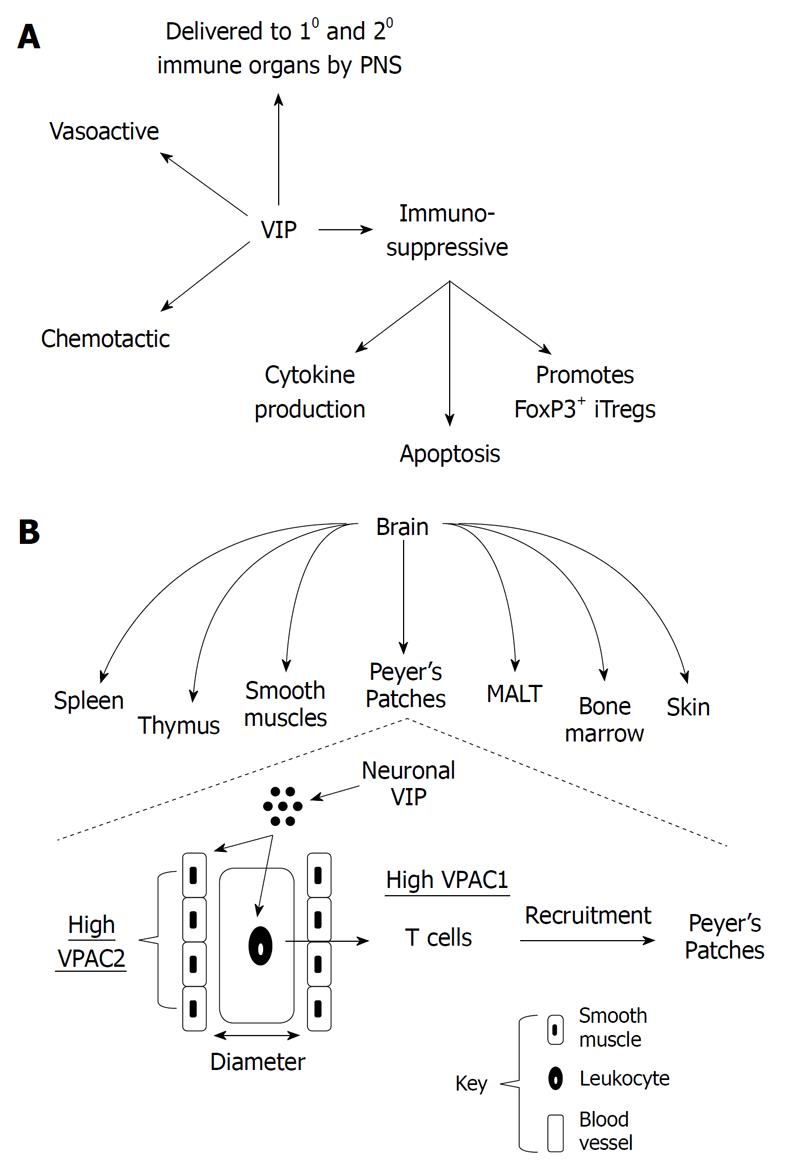
Figure 1 Neuroimmunomodulation by vasoactive intestinal peptide.
A: Vasoactive intestinal peptide (VIP) is delivered to primary (10) and secondary (20) immune organs by the peripheral nervous system (PNS), which affects the metabolism of cells in close proximity through its vasoactive properties (vascular smooth muscle cells) and its chemotactic activities on resting T lymphocytes. During TCR signaling, VIP is immunosuppressive directly on T lymphocytes by: inhibiting proinflammatory cytokine secretion/production, inhibiting apoptosis and promoting FoxP3+ inducible T regulatory cells; B: Delivery of VIP ligand to immune cells in indicated anatomical compartments (immune and non-immune) that represents a division of labor for VIP/vasoactive intestinal peptide receptor (VPAC)2 signaling (vasoactive; smooth muscle cells) and VIP/VPAC1 signaling (chemotactic and immunosuppressive; directly on lymphocytes) in an effort to effectively target trafficking naïve T cells to appropriate immune compartments such as Peyer’s Patches within the gut. MALT: Mucosa associated lymphoid tissue.
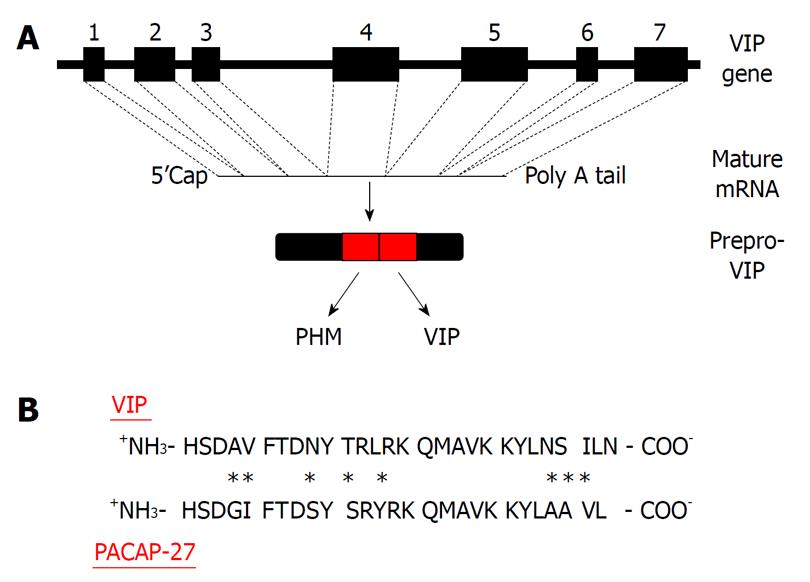
Figure 2 Molecular biology of vasoactive intestinal peptide.
A: Vasoactive intestinal peptide (VIP) is transcribed from a gene consisting of 7 exons and translated into a 170 amino acid prepropeptide that produces at least two biologically active peptides as shown; B: The amino acid comparison between pituitary adenylate cyclase activating polypeptide (PACAP)27 and VIP with bold letters representing identical amino acids between peptides, and asterisks indicating amino acid differences.
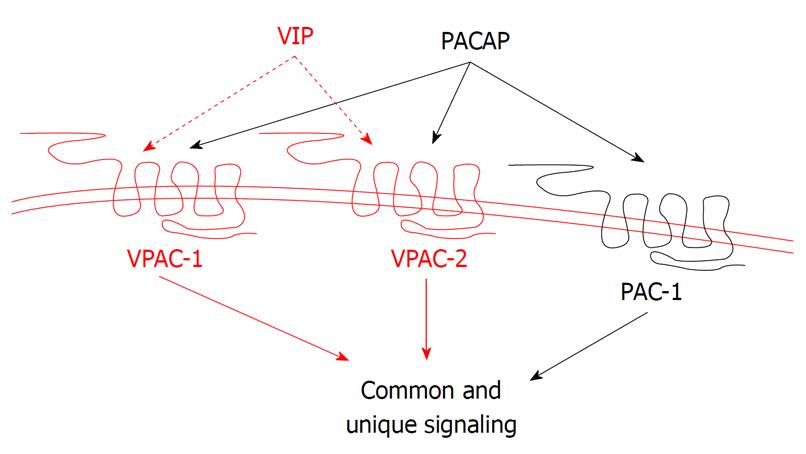
Figure 3 Binding selectivity of the vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide receptors.
Pituitary adenylate cyclase activating polypeptide receptor 1 (PAC1) selectively binds pituitary adenylate cyclase activating polypeptide (PACAP) with 1000-fold greater affinity than vasoactive intestinal peptide (VIP), whereas vasoactive intestinal peptide receptor (VPAC)1 and VPAC2 bind VIP and PACAP with equal affinity.
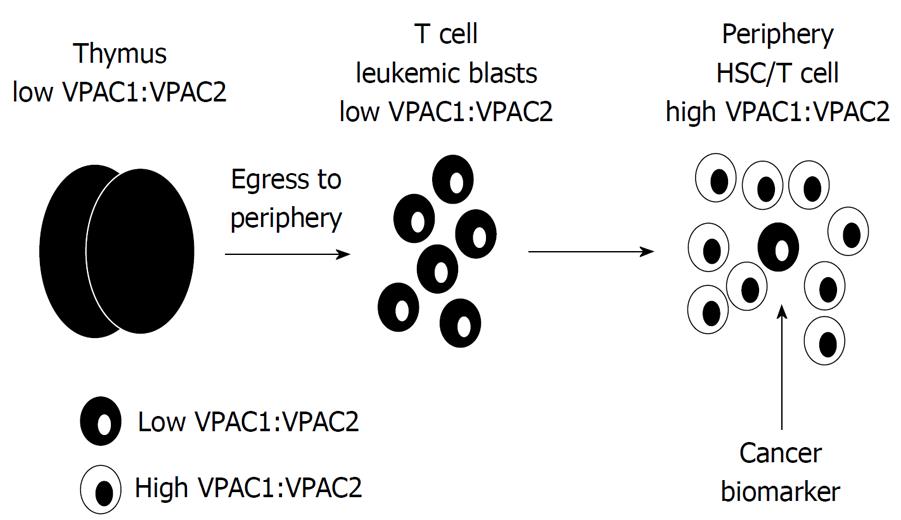
Figure 4 Working hypothetical model for differential vasoactive intestinal peptide receptor expression in T cell acute lymphoblastic leukemia blasts.
The radical difference between low vasoactive intestinal peptide receptor (VPAC)1:VPAC2 ratio in developing thymocytes may act as a biomarker and prognostic indicator, readily distinguishable from peripheral hematopoietic stem cells (HSC) and mature T cells that express high VPAC1:VPAC2 ratios.
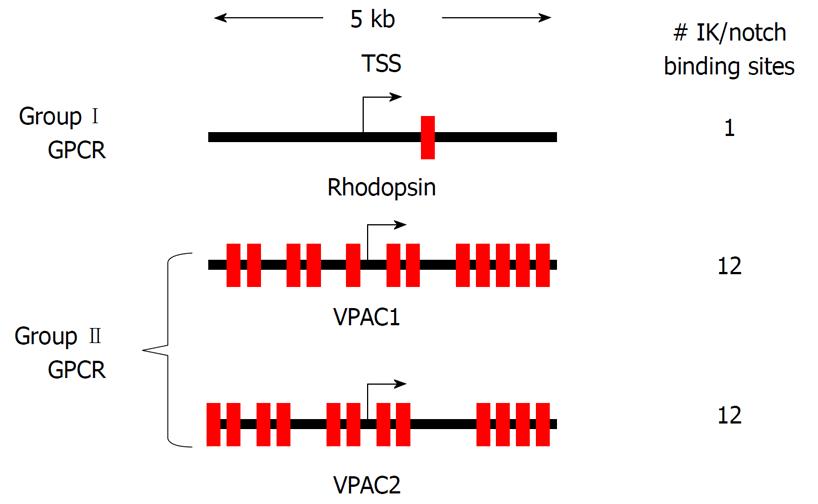
Figure 5 High Frequency IK binding sites at the vasoactive intestinal peptide receptor promoters.
Schematic diagram of a 5 kb region for the vasoactive intestinal peptide receptor (VPAC)1 and VPAC2 promoters spanning the transcriptional start site (TSS). Red boxes are IK consensus sequences, with the number of IK binding sites per gene promoter indicated.
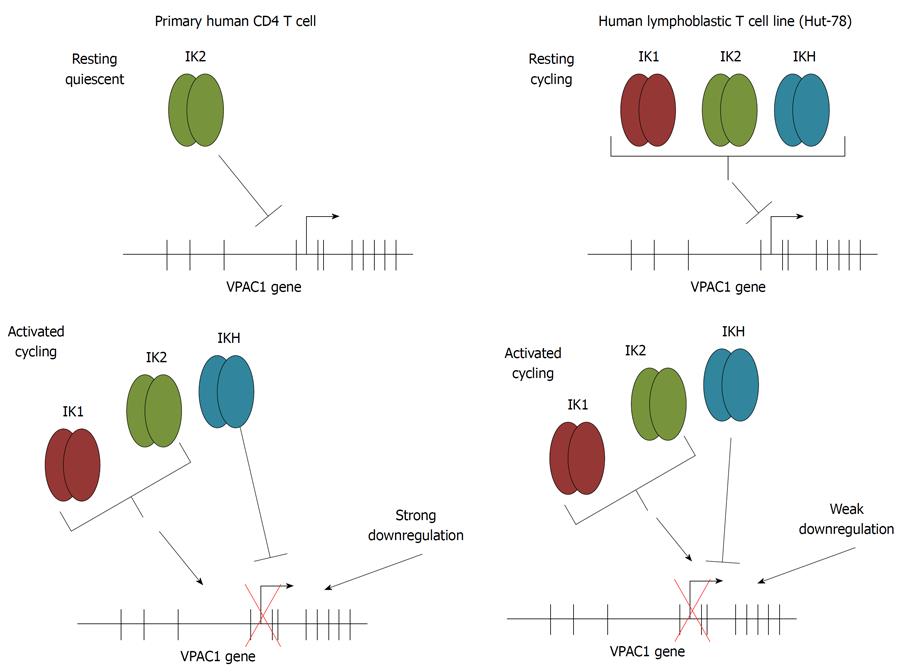
Figure 6 Ikaros engagement of the vasoactive intestinal peptide receptor 1 promoter in primary and T cell lines.
Schematic representation of electrophoretic mobility shift assays and chromatin immunoprecipitation assays data comparing Ikaros engagement to the vasoactive intestinal peptide receptor (VPAC)1 promoter in primary CD4 T cells compared to the human lymphoblastic T cell line, Hut-78 cells. Top panels represent the expression profile for Ikaros in resting cells, and the bottom panels represent activated T cells.
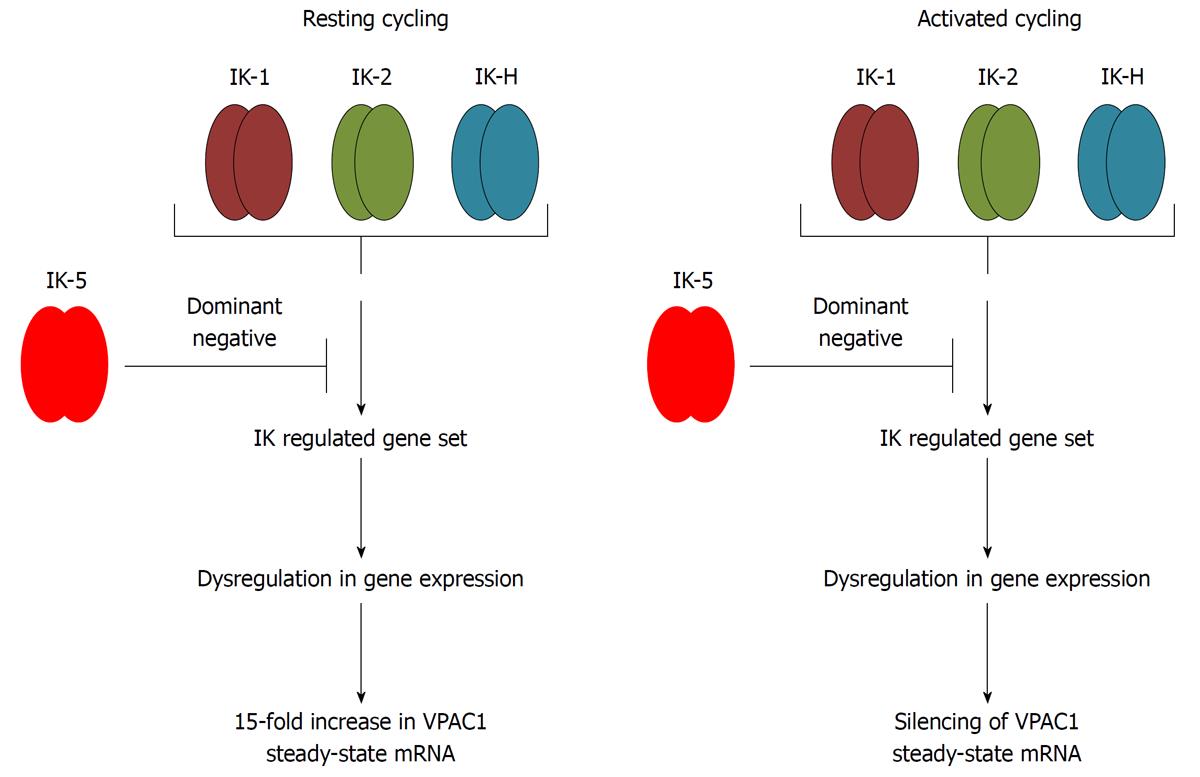
Figure 7 Working model for Ikaros mediated regulation of vasoactive intestinal peptide receptor 1 expression.
IK-5 overexpression results in the upregulation of vasoactive intestinal peptide receptor (VPAC)1 steady-state levels in resting Hut-78 cells. The DNA binding activity of Ikaros therefore can alter the expression of growth modulating genes like VPAC1 in a direct (binding to the VPAC1 gene) or indirect mechanism (regulating a repressor/activator that binds the VPAC1 gene). We predict VPAC1 silencing will occur in activated IK-5 overexpressing cells.
- Citation: Dorsam GP, Benton K, Failing J, Batra S. Vasoactive intestinal peptide signaling axis in human leukemia. World J Biol Chem 2011; 2(6): 146-160
- URL: https://www.wjgnet.com/1949-8454/full/v2/i6/146.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i6.146