Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2011; 2(5): 73-89
Published online May 26, 2011. doi: 10.4331/wjbc.v2.i5.73
Published online May 26, 2011. doi: 10.4331/wjbc.v2.i5.73
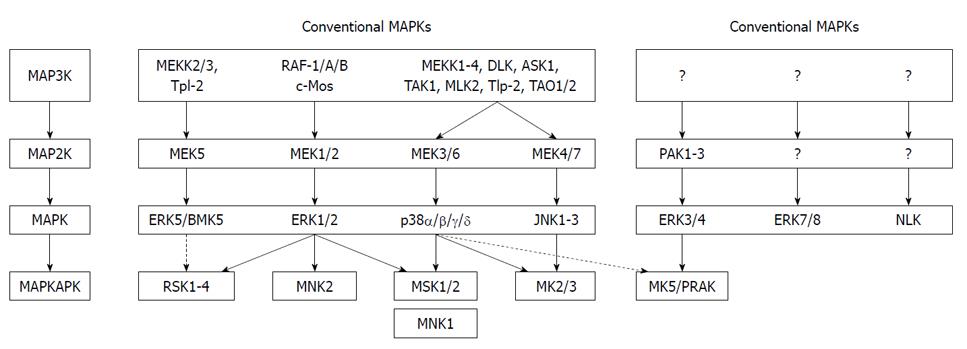
Figure 1 Mammalian mitogen-activated protein kinase pathways.
The conventional mitogen-activated protein kinase (MAPK) pathway has a tripartite composition in which MAPK kinase kinase (MAP3K) phosphorylates and activates MAPK kinase (MAP2K), which in turn phosphorylates and activates MAPK. The atypical MAPK pathway lacks this typical three-tiered cascade. Both conventional and atypical MAPKs can phosphorylate different substrates, including other protein kinases referred to as MAPK-activated protein kinases (MAPKAPKs). The stippled lines indicate that a bona fide in vivo link between these MAPKs and their MAPKAPKs remains controversial. ERK: Extracellular signal-regulated kinase; RSK: Ribosomal-S6-kinase; MNK: MAPK-interacting kinase; JNK: c-JUN N-terminal kinase; PAK: p21-activated protein kinase; MK: MAPK-activated protein kinase; NLK: Nemo-like kinase; PRAK: p38-regulated/activated protein kinase.
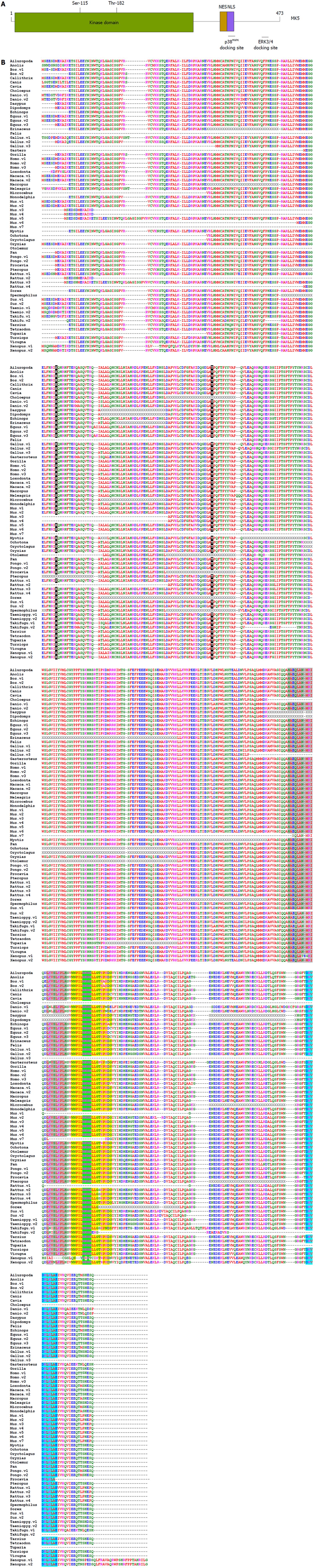
Figure 2 Structure of mitogen-activated protein kinase-activated protein kinase 5.
A: Functional domains of mitogen-activated protein kinase-activated protein kinase 5 (MK5); B: Primary sequence of annotated and proven MK5. The p38MAPK docking motif is in yellow, the nuclear localization signal (NLS) is shaded in green, the protein kinase A inhibitor (PKI)-like and REV-like nuclear export signal (NES) motifs are depicted in green, and the extracellular signal-regulated kinases (ERK)3/4 interaction domain is shaded in light blue. Notice that the NLS and p38MAPK docking site are overlapping. Threonine residue 182 in the activation loop and the protein kinase A phosphoacceptor site Ser-115 are highlighted in black. The one-letter amino acid code is used. Red represents small and hydrophobic amino acids (A, V, F, P, M, I, L, W), blue symbolizes acidic amino acids (D, E), magenta corresponds to basic amino acids (R, K), while green stands for hydroxyl, amine and basic amino acids (S, T, Y, C, N, G, Q, H). Non-annotated residues are indicated by X, while gaps are shown by dashed lines. The abbreviation v after the name of the organism refers to different isoforms. Clustal series of programs was used for multiple sequence alignment[67].
Figure 3 Amino acid alignment of the of the phosphoacceptor site motifs in mitogen-activated protein kinase-activated protein kinase 5 substrates.
The phosphoacceptor site is underlined. The synthetic substrate peptide PRAKtide, which is derived from glycogen synthatese[40], and the peptide substrate derived from the regulatory light chain of myosin II[3] are also shown.
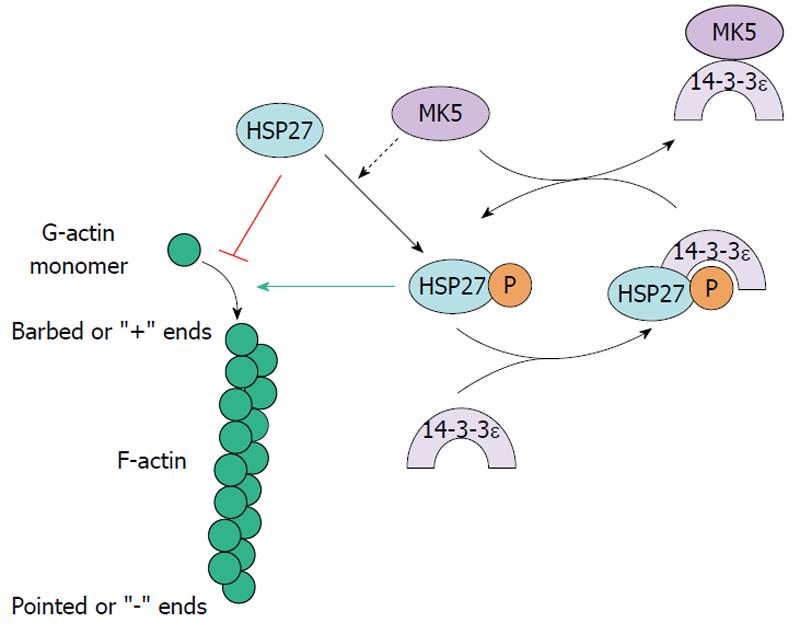
Figure 4 Mitogen-activated protein kinase-activated protein kinase 5 and F-actin remodeling through Hsp27.
Mitogen-activated protein kinase-activated protein kinase 5 (MK5) can phosphorylate HSP27 and this stimulates F-actin polymerization. 14-3-3ε can bind and inhibit the enzymatic activity of MK5 and thus prevent F-actin remodeling. 14-3-3ε has also been shown to bind phosphorylated HSP27. MK5 may extricate 14-3-3ε from pHSP27 and releasing pHSP27 which in turn can mediate F-actin polymerization.
- Citation: Kostenko S, Dumitriu G, Lægreid KJ, Moens U. Physiological roles of mitogen-activated-protein-kinase-activated p38-regulated/activated protein kinase. World J Biol Chem 2011; 2(5): 73-89
- URL: https://www.wjgnet.com/1949-8454/full/v2/i5/73.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i5.73