Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Apr 26, 2011; 2(4): 67-72
Published online Apr 26, 2011. doi: 10.4331/wjbc.v2.i4.67
Published online Apr 26, 2011. doi: 10.4331/wjbc.v2.i4.67
Figure 1 Emanuel E Strehler, PhD, Professor of Biochemistry and Molecular Biology, Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine, 200 First Street S.
W., Rochester, MN 55905, United States.
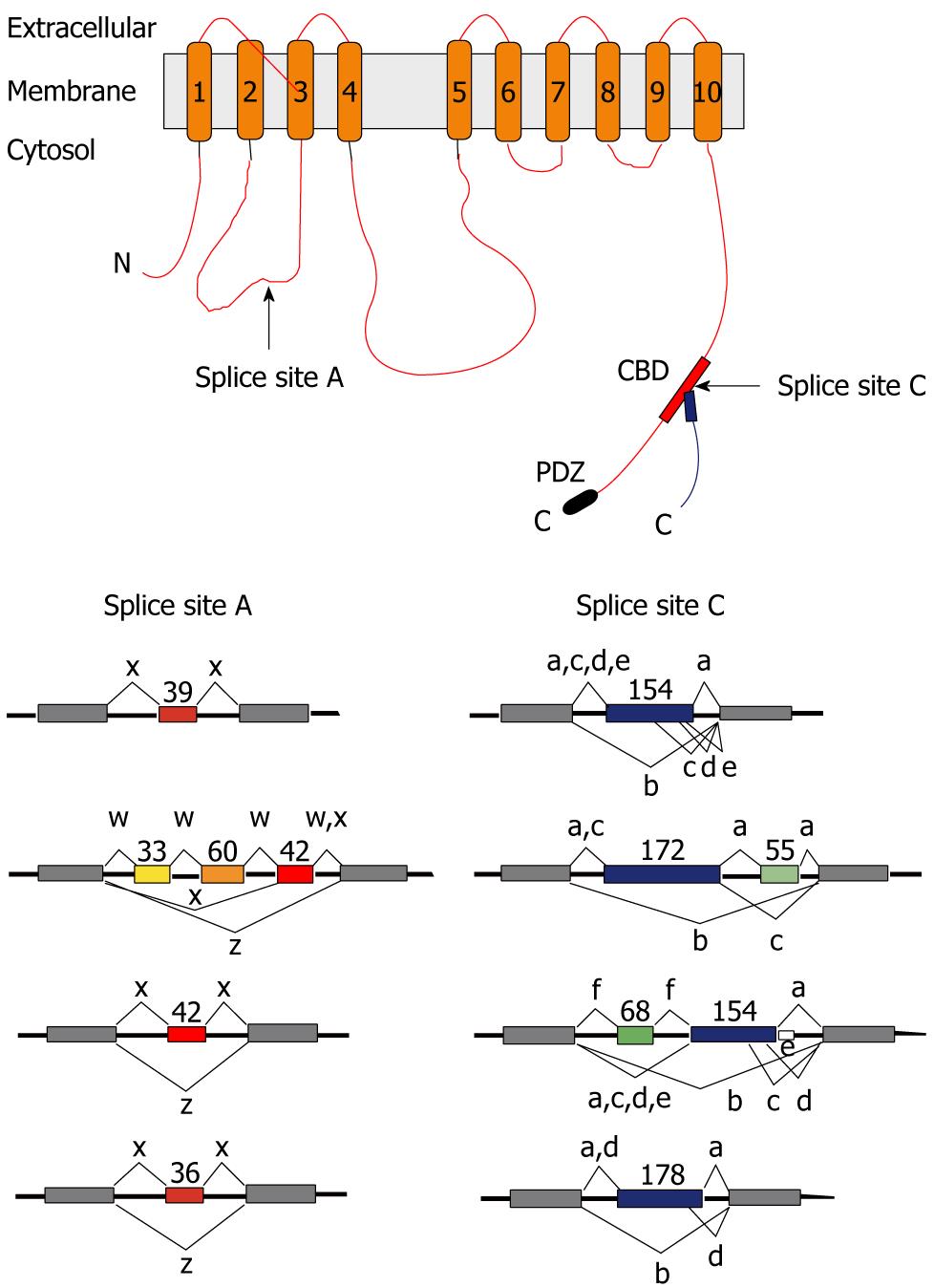
Figure 2 Scheme of the plasma membrane calcium pump and of the alternative splice options generated from the four plasma membrane calcium pump genes.
Top: Scheme of the plasma membrane calcium pump (PMCA). The transmembrane regions are numbered 1-10, and arrows indicate the sites affected by alternative splicing. Splicing at site C affects the calmodulin-binding domain (CBD) and can result in different C tails due to a shift in reading frame (indicated as separate lines). Only splice variants of the “b-type” carry a PDZ domain recognition sequence (PDZ) at the C terminus. N: N terminus; C: C terminus; Bottom: Exon structure of the regions involved in alternative splicing in the four human PMCA genes. Constitutively spliced exons are shown as gray boxes. The sizes of alternatively spliced exons (colored) are given in nucleotides, the alternative splicing options are indicated by connecting lines, and the resultant splice products are labeled by their lower case symbol. Combinatorial use of the splice options at sites A and C yields over 30 possible PMCA splice variants.
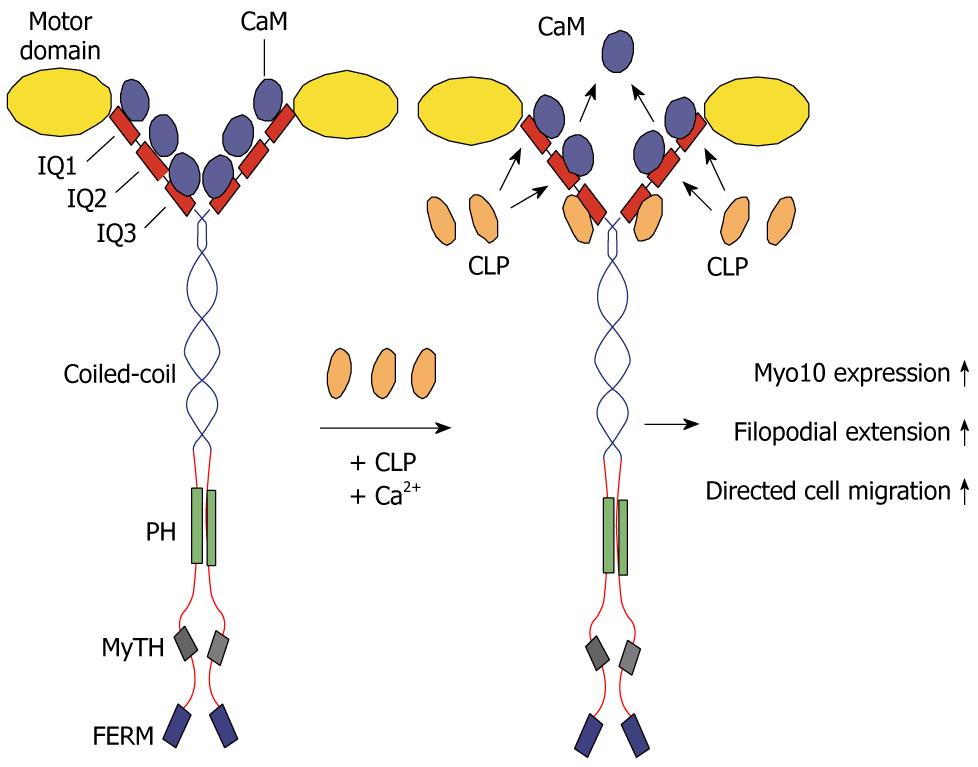
Figure 3 Calmodulin-like protein serves as light chain for Myo10 and increases its expression and function.
Myo10 consists of two heavy chains with an N-terminal motor domain, a “neck” region of three IQ domains involved in light chain binding, a putative coiled-coil region, and a tail region that contains pleckstrin homology (PH) domains, a myosin tail homology (MyTH) domain, and a C-terminal FERM (4.1, ezrin, radixin, moesin) domain. The IQ motifs bind calmodulin (CaM) or calmodulin-like light chains, which regulate Myo10 activity and Ca2+ sensitivity. Upon expression of calmodulin-like protein (CLP) (in the presence of Ca2+), it competes successfully with calmodulin and binds tightly to IQ3 and possibly IQ1 and IQ2 of Myo10. CLP binding results in increased Myo10 expression and function, as demonstrated by increased filopodial extension and enhanced directional cell migration.
- Citation: Strehler EE. Emanuel Strehler’s work on calcium pumps and calcium signaling. World J Biol Chem 2011; 2(4): 67-72
- URL: https://www.wjgnet.com/1949-8454/full/v2/i4/67.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i4.67