Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Nov 26, 2011; 2(11): 246-251
Published online Nov 26, 2011. doi: 10.4331/wjbc.v2.i11.246
Published online Nov 26, 2011. doi: 10.4331/wjbc.v2.i11.246
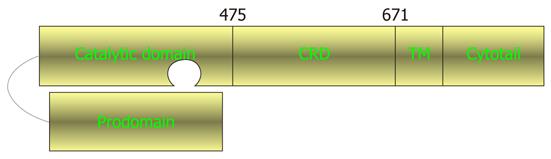
Figure 1 Schematic drawing of tumor necrosis factor-α converting enzyme domain structure.
Lengths of domains are not in proportion. The amino acids marking the beginning and the end of the cysteine-rich/disintegrin domain (CRD) are numbered. TM: Transmembrane domain.
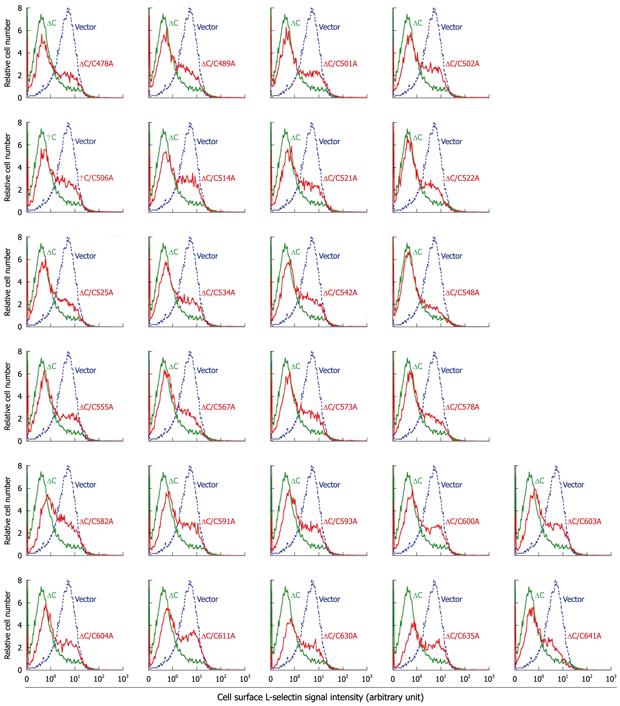
Figure 2 L-selectin ectodomain shedding activity in cytotail-deleted tumor necrosis factor-α converting enzyme constructs with cysteine to alanine substitutions.
The shedding-defective M1-L-selectin cells, which stably express transmembrane L-selectin, were transiently cotransfected with the transfection marker green fluorescence protein (GFP), and indicated tumor necrosis factor-α converting enzyme (TACE) vectors or the control pRK5 plasmid at a ratio of 1:3. Transmembrane L-selectin on the surface of live unfixed cells was immunostained with anti-L-selectin and a PE-conjugated secondary antibody, and was detected by flow cytometry. GFP-positive cells were gated as cells expressing the cotransfected TACE construct. Green: ∆C TACE; Red: ∆C TACE with a cysteine to alanine substitution at indicated position; dotted blue lines, pRK5.
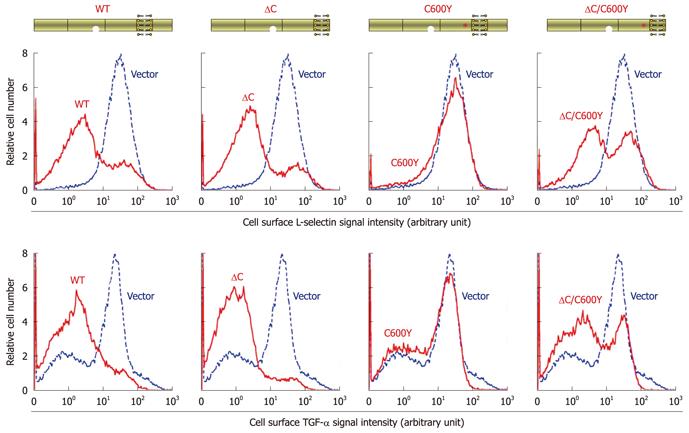
Figure 3 Deletion of the cytotail from C600Y tumor necrosis factor-α converting enzyme led to partial restoration of L-selectin and transforming growth factor α shedding.
Expression plasmids for wild-type or mutated tumor necrosis factor-α converting enzyme (TACE) are schematically shown on the top of the Figure. The activity of L-selectin shedding in the TACE constructs was determined as in Figure 2. Transforming growth factor (TGF)-α shedding was determined in a similar manner except the M1-L-selectin cells were replaced with shedding-defective M1-TGF-α cells, which overexpress transmembrane TGF-α, and the anti-L-selectin was substituted with anti-TGF-α.
- Citation: Li X, Pérez L, Fan H. Inhibitory role of TACE/ADAM17 cytotail in protein ectodomain shedding. World J Biol Chem 2011; 2(11): 246-251
- URL: https://www.wjgnet.com/1949-8454/full/v2/i11/246.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i11.246