Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Oct 26, 2011; 2(10): 215-225
Published online Oct 26, 2011. doi: 10.4331/wjbc.v2.i10.215
Published online Oct 26, 2011. doi: 10.4331/wjbc.v2.i10.215
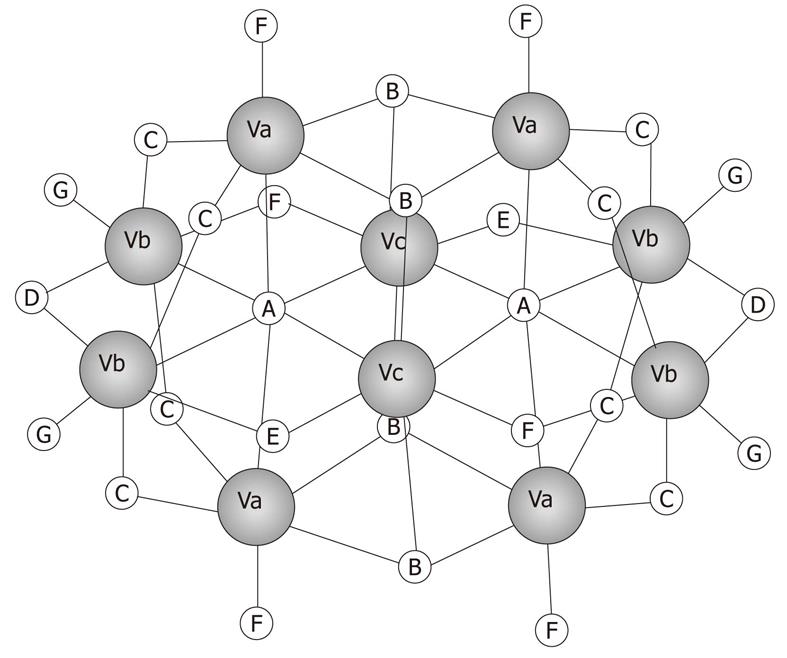
Figure 1 Schematic structure of V10 (V10O286-).
Va, Vb and Vc represent the three different types of vanadium atoms described in the text.
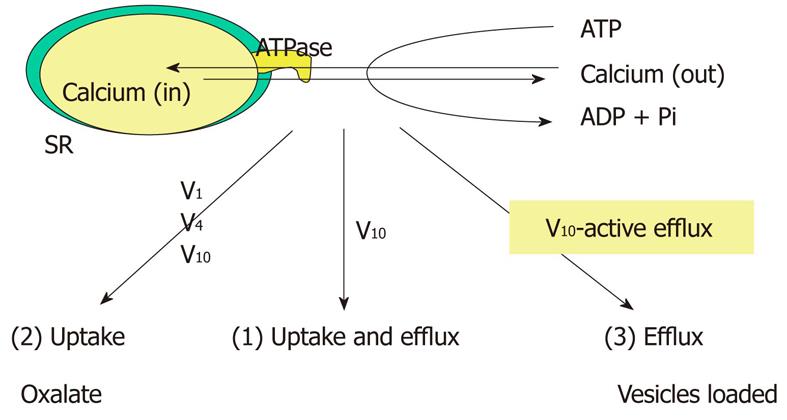
Figure 2 Modes of calcium translocation by SR calcium pump as affected by different vanadate oligomers.
V1: Monomeric vanadate; V4: Tetrameric vanadate; V10: Decameric vanadate. Only V10, and not V1, was shown to inhibit calcium uptake in conditions 1 and 3, that is, when ATPase activity is coupled to calcium transport. V1 only inhibits the ATPase in condition 2, where the calcium gradient is destroyed by oxalate or phosphate.
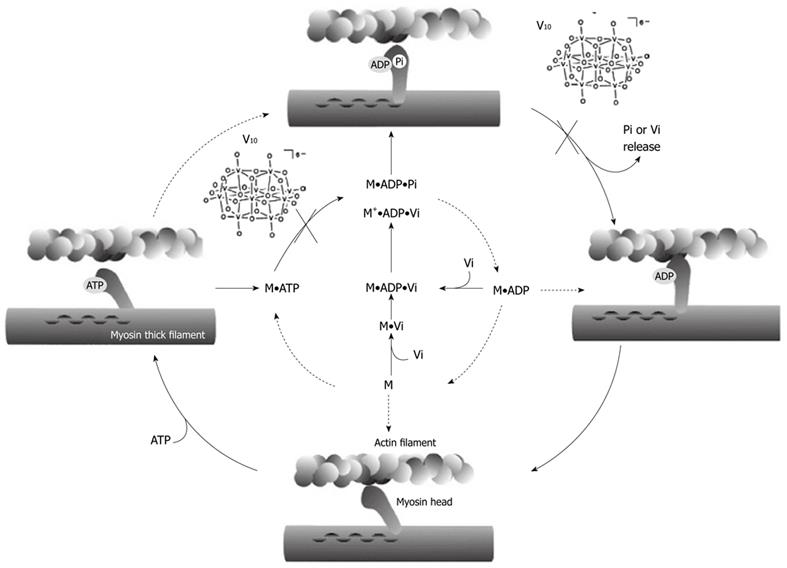
Figure 3 Relevant steps of ATP hydrolysis by actomyosin complex.
The dominant process of ATP hydrolysis observed in vitro is indicated by the filled arrows. M: Myosin; Vi: Orthovanadate; V10: Decavanadate. V10, can blocked the process at two steps, without or with F-actin: before ATP hydrolysis and before product release, just before power stroke.
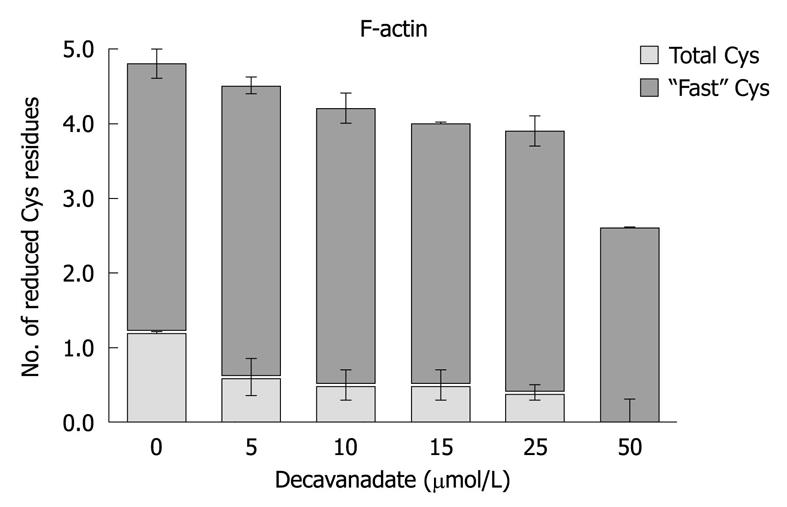
Figure 4 F-actin cysteine redox state, after 20 min exposure to decavanadate.
Titration of cysteine was performed with 0.1 mmol/L 5,5’-ditiobis-(2-nitrobenzoic acid) and 2 μmol/L actin in 2 mmol/L Tris (pH 7.5), and 0.2 mmol/L CaCl2. The increase in absorbance at 412 nm was continuously recorded over 10 min; To measure total cysteines the samples were treated afterwards with 1% SDS, and the absorbance was measured, over 15-30 min, until a steady value was reached. Titration with decavanadate produced a dose-dependent decrease in F-actin total cysteines, while Cys-374 (also named "fast cysteine") is reduced. The results shown are the average of triplicate experiments.
Figure 5 Exchange of bound ε-ATP of G-actin with ATP.
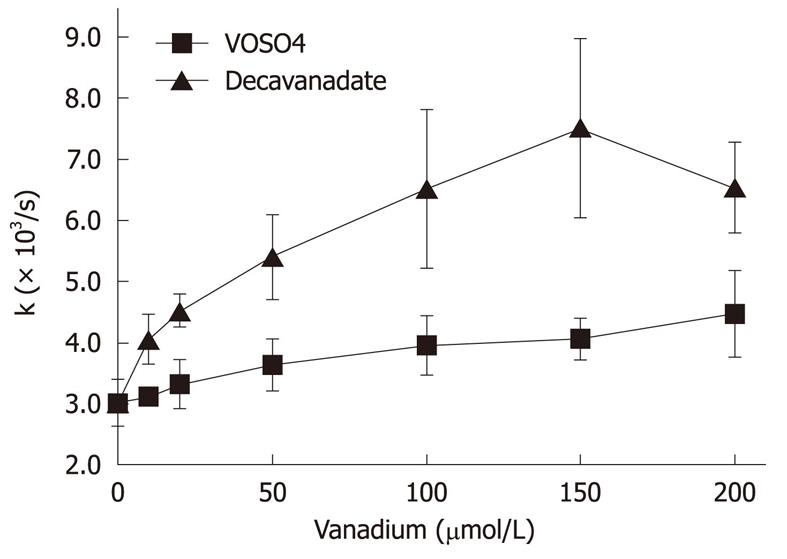
Actin monomers (5 μmol/L) were incubated for 20 min with 0-200 μmol/L decavanadate, in 2 mmol/L Tris-HCl (pH 7.5), 0.2 mmol/L CaCl2. The nucleotide exchange was monitored by the fluorescence decrease (λex = 350 nm; λem = 410 nm), as ε-ATP was replaced by ATP. Data are plotted as mean ± SD. The results shown are the average of triplicate experiments.
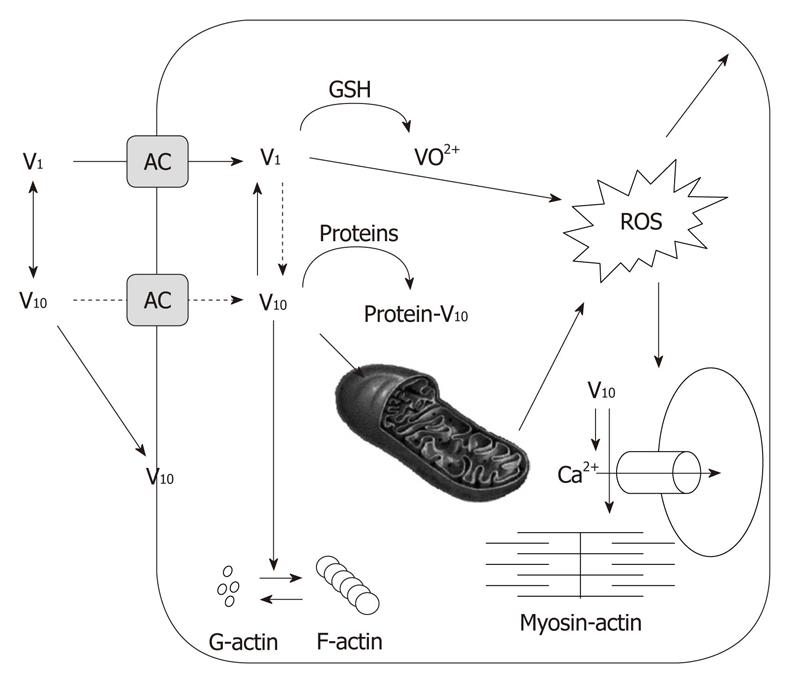
Figure 6 Scheme of proposed decavanadate (V10) cellular targets.
V10 uptake through anionic channels (AC). Decavanadate might interact with membrane proteins. V10 formation upon intracellular vanadium acidification in cytosol, but most probably in acidic organelles. Reduction of monomeric vanadate (V1) by antioxidant agents. Binding of V10 to target proteins; it is proposed that V10 accumulates in subcellular organelles, such as mitochondria, affecting its function. Decavanadate also targets the contractile system, and its regulation, as well as calcium homeostasis (adapted from[14]).
- Citation: Aureliano M, FCT, Algarve UO, Gambelas, Faro 81, Portugal. Recent perspectives into biochemistry of decavanadate. World J Biol Chem 2011; 2(10): 215-225
- URL: https://www.wjgnet.com/1949-8454/full/v2/i10/215.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i10.215