Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Aug 26, 2010; 1(8): 239-247
Published online Aug 26, 2010. doi: 10.4331/wjbc.v1.i8.239
Published online Aug 26, 2010. doi: 10.4331/wjbc.v1.i8.239
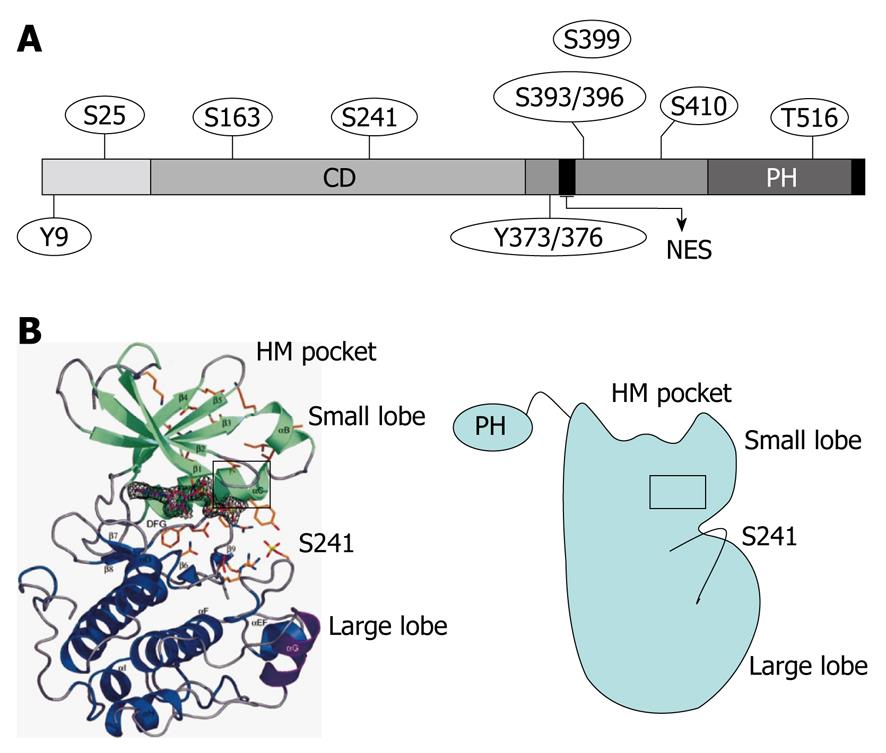
Figure 1 Secondary structure and phosphorylation sites of 3-phosphoinositide-dependent protein kinase-1.
A: 3-phosphoinositide-dependent protein kinase-1 (PDK1) consists of an N-terminal kinase catalytic domain (CD; amino acids 71-359) and a C-terminal pleckstrin homology (PH) domain (amino acids 459-550). The nuclear export sequence (NES) (amino acids 379-388) is essential for exporting PDK1 into the cytoplasm from the nucleus. The phosphorylation sites of PDK1 include Ser-241 and Tyr-373/376, which is dependent on Tyr-9 and required for PDK1 catalytic activity; B: Crystal structure of the human PDK1 kinase domain. Residues 71-163 are green (small lobe) and residues 164-358 are blue (large lobe). The αC-helix (amino acids 124-136) is boxed in black and encompasses residues 287-295, which are purple. The hydrophobic motif (HM) pocket with the Ser-241 in the activation loop is also shown. This figure was adopted from Biondi et al[26], 2002.
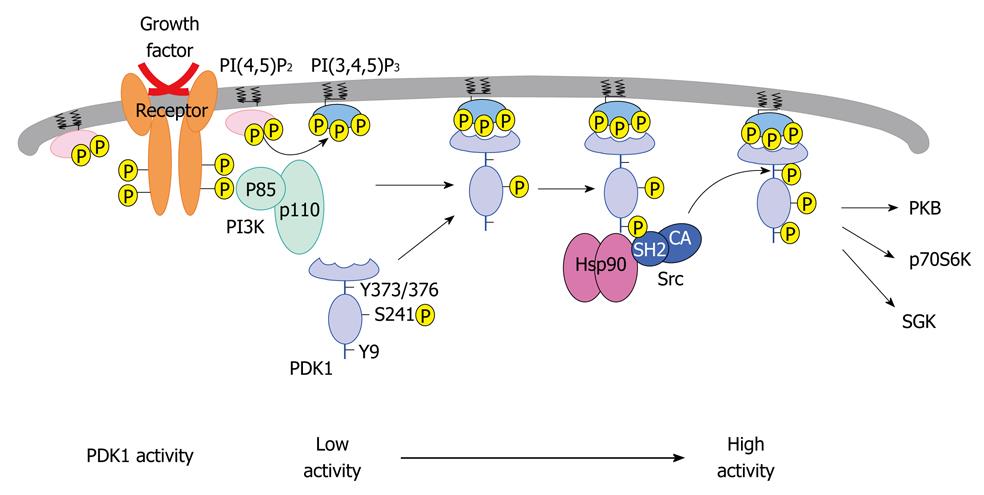
Figure 2 Proposed mechanism for the regulation of 3-phosphoinositide-dependent protein kinase-1 phosphorylation and stability.
3-phosphoinositide-dependent protein kinase-1 (PDK1) autophosphorylates itself on Ser-241. In the presence of pervanadate or insulin, PDK1 is phosphorylated on Tyr-9 and Tyr-373/376 with the help of Src and heat shock protein 90 (Hsp90). Tyrosine phosphorylation further increases PDK1 catalytic activity. In the absence of Hsp90 interaction, PDK1 is promoted towards proteasome-dependent degradation. This figure was adopted from Yang et al[41], 2008. PI3K: Phosphoinositide 3-kinase; PI(4,5)P2: Phosphatidylinositol 3,4 bisphosphate; PI(3,4,5)P3: Phosphatidylinositol 3,4,5 trisphosphate; CA: Constitutively active; PH: Pleckstrin homology; PKB: Protein kinase B; SGK: Serum and glucocorticoid-inducible kinase.
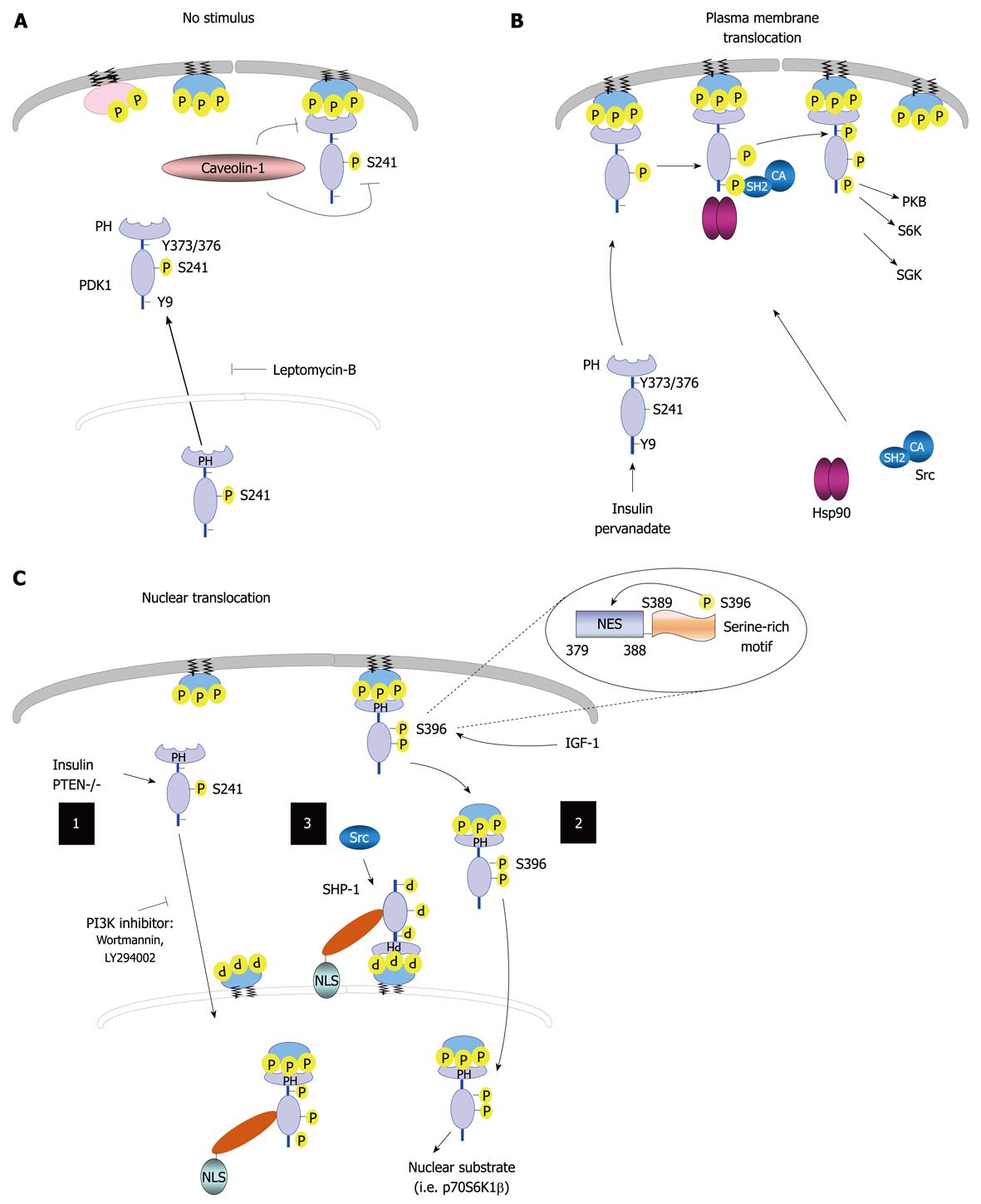
Figure 3 Regulation of 3-phosphoinositide-dependent protein kinase-1 cellular localization.
A: 3-phosphoinositide-dependent protein kinase-1 (PDK1) resides in the cytoplasm and membranes in unstimulated cells, and can shuttle between these two compartments. The treatment of cells with leptomycin-B results in PDK1 accumulation in the nucleus. The localization of this kinase to the plasma membrane is disrupted by caveolin-1; B: PDK1 can translocate to the plasma membrane following treatment with pervanadate or insulin, which leads to PDK1 phosphorylation on Tyr-9 and Tyr-373/376; C: Nucleus-cytoplasm shuttle. (1) Phosphoinositide 3-kinase (PI3K) signaling regulates PDK1 nuclear translocation; (2) Insulin growth factor-1 (IGF-1) induces Ser-396 phosphorylation, thereby placing the serine-rich motif (amino acids Ser-389-396) close to its nuclear export sequence (NES) region, which leads to PDK1 nuclear translocation; and (3) Tyrosine phosphatase SH2 domain-1 (SHP-1) binds to PDK1 following tyrosine phosphorylation. The SHP-1/PDK1 complex is recruited to the nuclear membrane by binding to perinuclear phosphatidylinositol 3,4,5 trisphosphate, where SHP-1 and its nuclear localization signal (NLS) facilitates active import. In addition, Src promotes the association of SHP-1 to PDK1. Nuclear PDK1 regulates its nuclear substrates and activates corresponding signaling pathways. PTEN: Phosphatase and tensin homolog; CA: Constitutively active; PH: Pleckstrin homology; PKB: Protein kinase B; SGK: Serum and glucocorticoid-inducible kinase; Hsp90: Heat shock protein 90.
- Citation: Li Y, Yang KJ, Park J. Multiple implications of 3-phosphoinositide-dependent protein kinase 1 in human cancer. World J Biol Chem 2010; 1(8): 239-247
- URL: https://www.wjgnet.com/1949-8454/full/v1/i8/239.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i8.239