Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2010; 1(5): 69-80
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.69
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.69
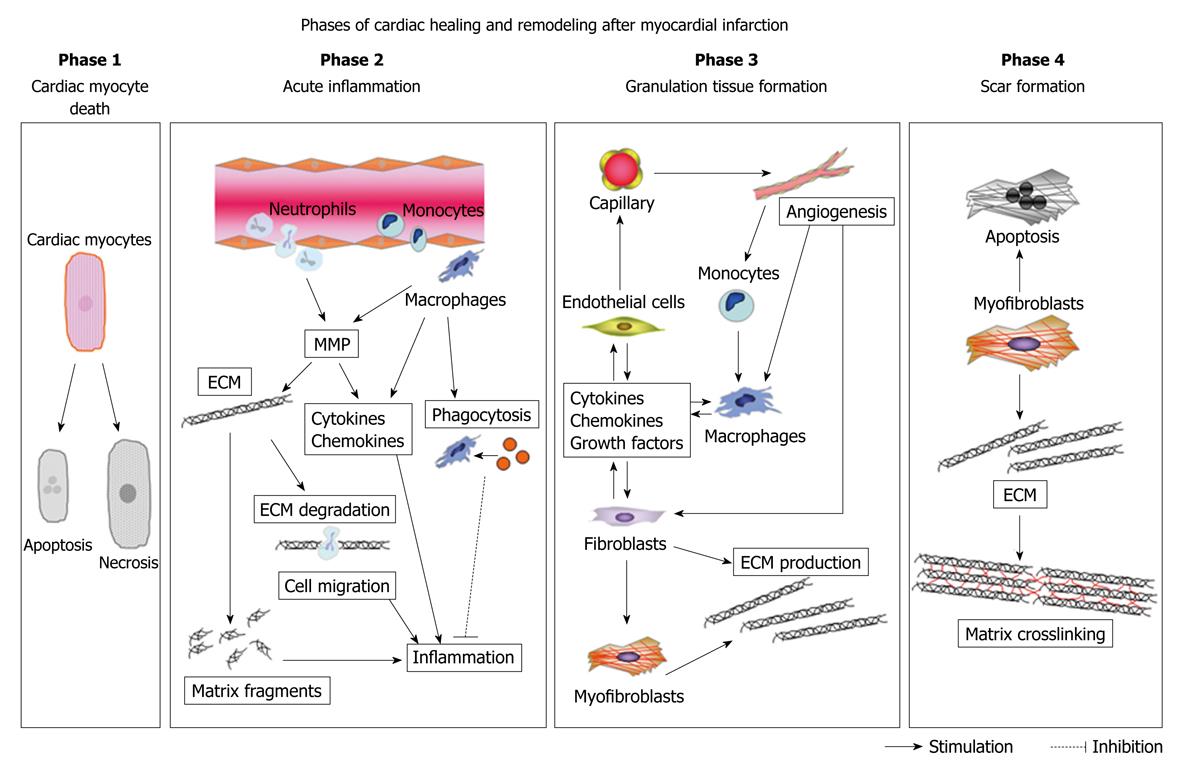
Figure 1 Phases of cardiac healing and remodeling after myocardial infarction (MI).
The cardiac healing and remodeling process after MI can be divided into four phases: (1) death of cardiomyocytes; (2) acute inflammation; (3) formation of granulation tissue; and (4) scar formation. Death of cardiomyocytes starts at approximately 1 h after coronary artery occlusion, and can either be the result of apoptosis or necrosis. During acute inflammation, the influx of inflammatory cells, including neutrophils and monocytes, for phagocytosis and removal of dead cardiomyocytes into the infarcted area and degradation of the extracellular matrix (ECM) by matrix metalloproteinase (MMP) takes place between 1 h and 4 d after MI. MMP also modulates inflammatory cytokine and chemokine activity. Generation of matrix fragments exerts potent inflammatory effects. Thereafter, formation of granulation tissue, characterized by the presence of fibroblasts, macrophages, myofibroblasts, new blood vessels, and ECM proteins, occurs in the infarcted heart between 2 and 14 d after MI. To rescue the loss of the shielding effects of the normal matrix, fibroblasts and myofibroblasts produce ECM. Finally, granulation tissue matures in the infarcted heart between 14 d and 2 mo after MI. The scar is characterized by a cross-linked, collagen-rich region that is induced by lysyl oxidase. In this phase, most infarct myofibroblasts undergo apoptosis and disappear. The time intervals for each phase are dependent on the species, as rodents exhibit an accelerated inflammatory and reparative response following MI as compared with large mammals.
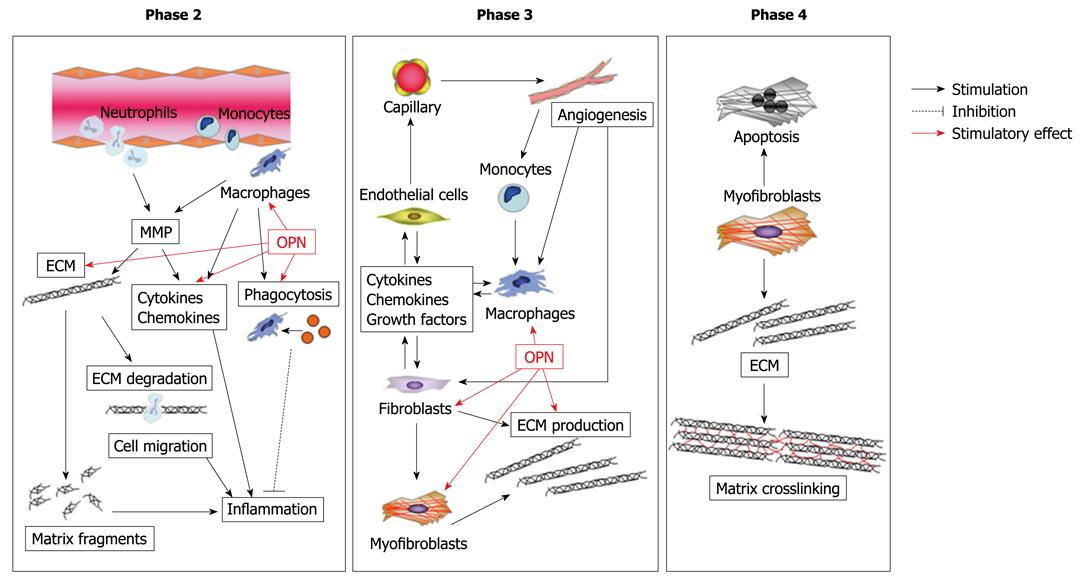
Figure 2 Possible role of osteopontin (OPN) in cardiac healing and remodeling after MI.
OPN modulates various functions of macrophages, such as migration, phagocytosis, and pro-inflammatory cytokine production. OPN also regulates proliferation and adhesion of fibroblasts. Moreover, OPN modulates myofibroblast differentiation and function, thereby promoting collagen synthesis and organization.
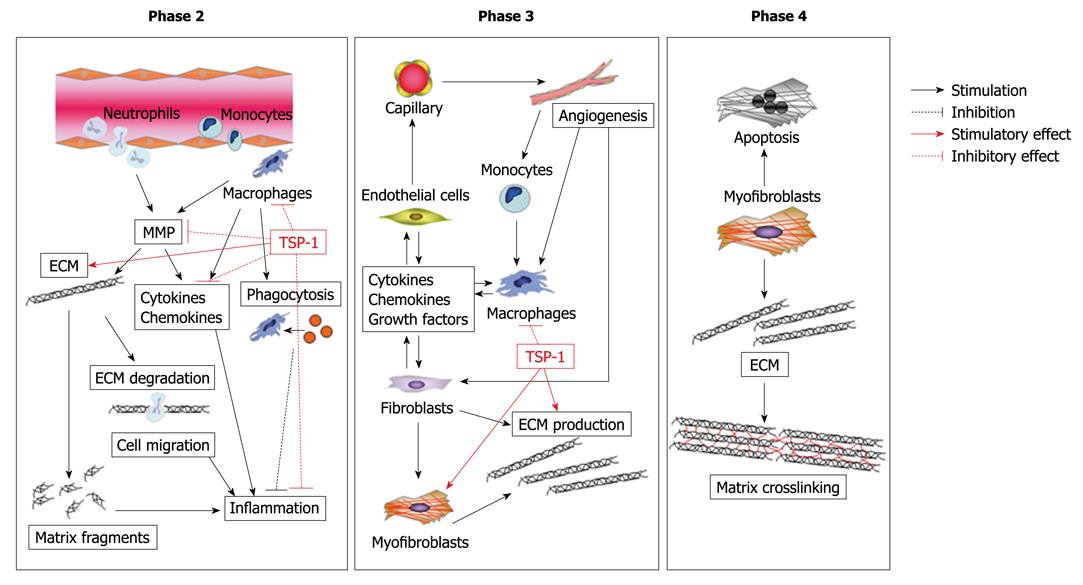
Figure 3 Possible role of thrombospondin-1 (TSP-1) in cardiac healing and remodeling after MI.
Expression of TSP-1 works as a barrier that suppresses inflammatory chemokines and cytokines via its transforming growth factor-β1 (TGF-β1)-activating effect, thereby preventing extension of the inflammatory process into the non-infarcted region. In addition, TSP-1 could affect maturation of the infarct scar through the activation of TGF-β1 and inhibition of MMP activity.
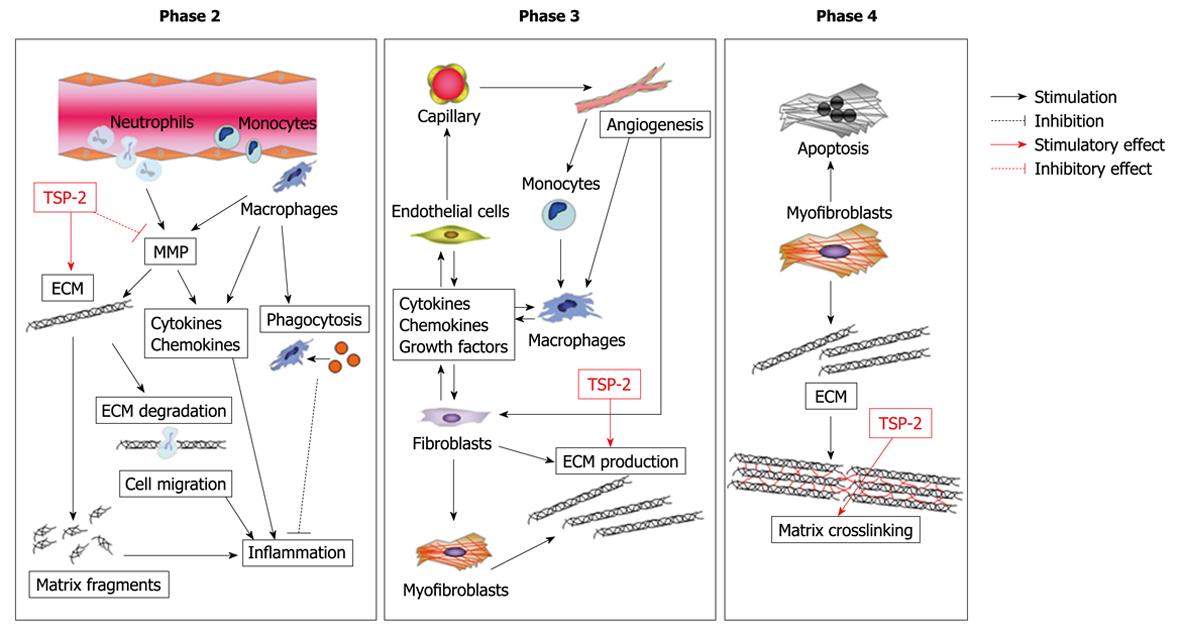
Figure 4 Possible role of TSP-2 in cardiac healing and remodeling after MI.
TSP-2 is a crucial regulator of the integrity of the cardiac ECM during maturation of the infarct scar, presumably via regulation of MMP activity, and subsequent deposition of a cross-linked collagen matrix.
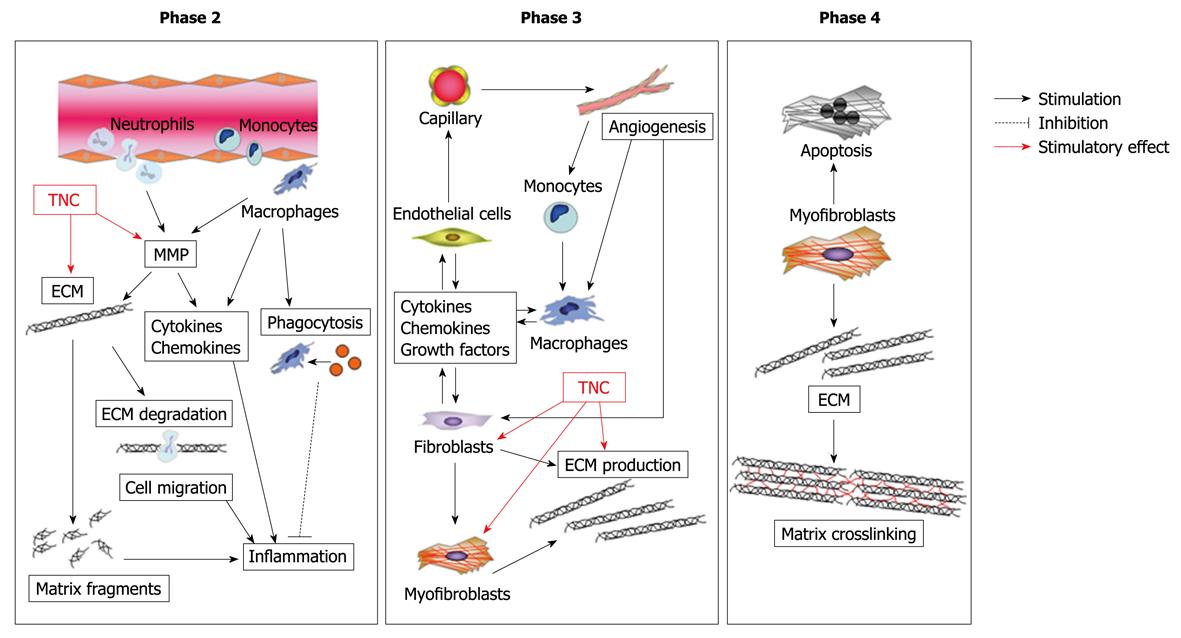
Figure 5 Possible role of tenascin-C (TNC) in cardiac healing and remodeling after MI.
TNC stimulates fibroblasts to produce matrix, thereby causing cardiac fibrosis and maladaptive remodeling after MI. Furthermore, TNC upregulates transcription and activity of MMP and promotes degradation of ECM.
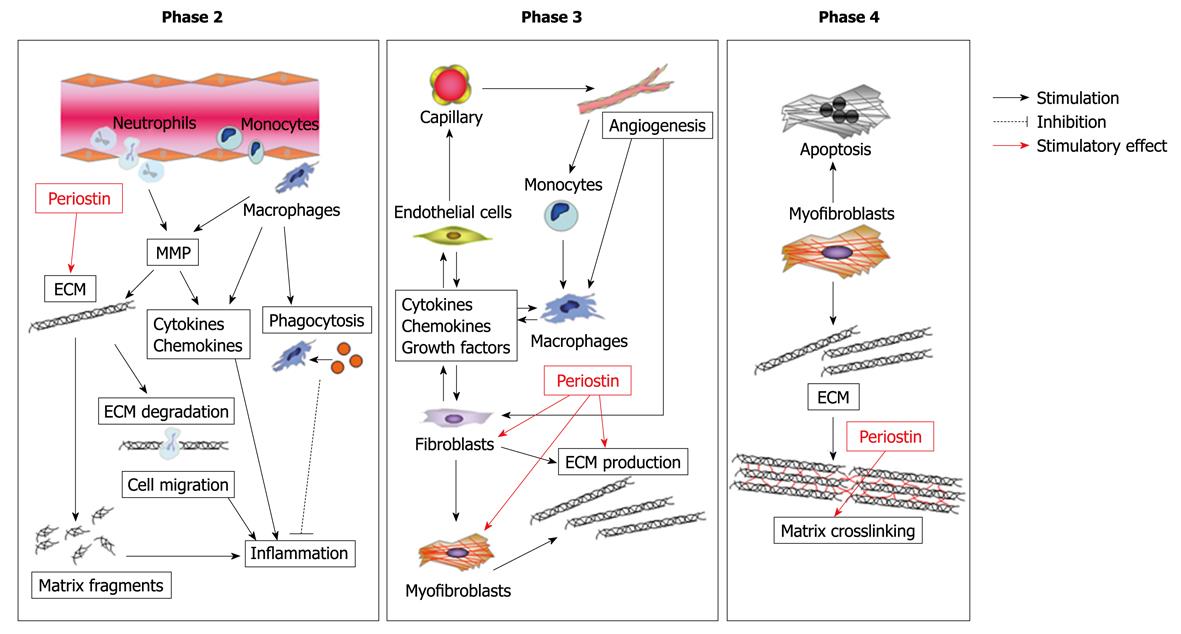
Figure 6 Possible role of periostin in cardiac healing and remodeling after MI.
Periostin regulates the integrity of the ECM through its ability to bind multiple ECM proteins, thereby affecting the structural integrity of the adult heart matrix. In addition, periostin promotes migration of fibroblasts via interaction with integrin.
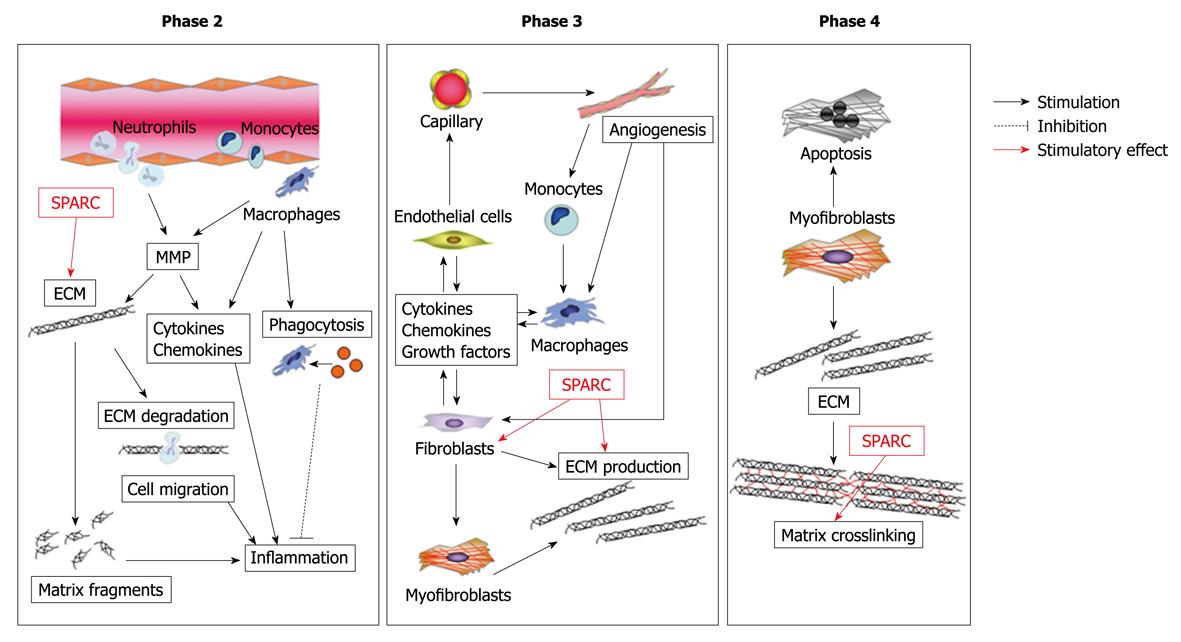
Figure 7 Possible role of secreted protein, acidic and rich in cysteine (SPARC) in cardiac healing and remodeling after MI.
SPARC regulates collagen maturation by direct interaction with collagen type I fibers and regulation of fibronectin matrix assembly. SPARC mediates TGF-β1 signaling, which is involved in wound healing and collagen production, thereby regulating cardiac healing and remodeling.
- Citation: Matsui Y, Morimoto J, Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J Biol Chem 2010; 1(5): 69-80
- URL: https://www.wjgnet.com/1949-8454/full/v1/i5/69.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i5.69