Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2010; 1(5): 196-200
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.196
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.196
Figure 1 Simon Labbé, PhD, Professor, Department of Biochemistry, Faculty of Medicine, Université de Sherbrooke, Canada.
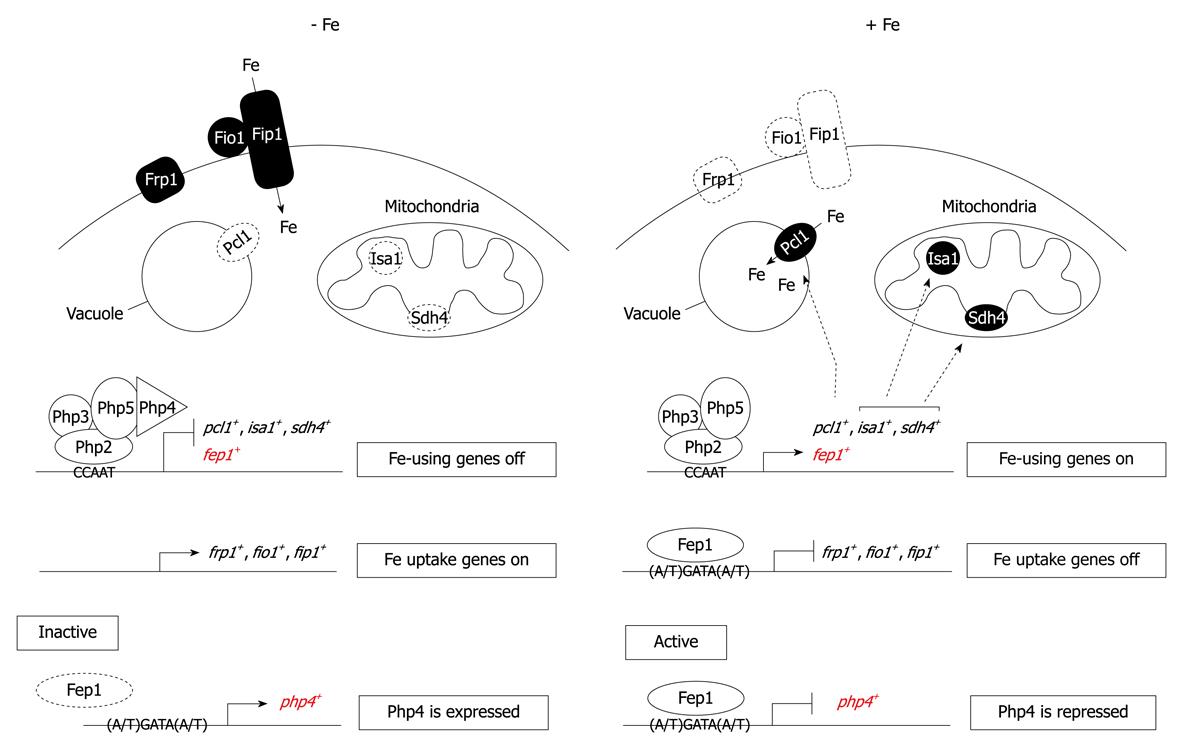
Figure 2 A proposed transcription model for an “iron economy”.
Under low iron supply conditions, Fep1 cannot bind to DNA and php4+ is expressed. Once biosynthesized, Php4 interacts with the Php2/Php3/Php5 heterotrimer to mediate repression of genes that encode iron-storage (Pcl1) and iron-containing (Sdh4 and Isa1) proteins, as well as the fep1+ gene. Conversely, under iron-replete conditions, Fep1 binds DNA and, with the aid of Tup11 and Tup12, turns off php4+ gene expression. This inactivation of php4+ enables genes involved in storage and utilization of iron to be expressed via the Php2/Php3/Php5 heterotrimeric CCAAT binding factor.
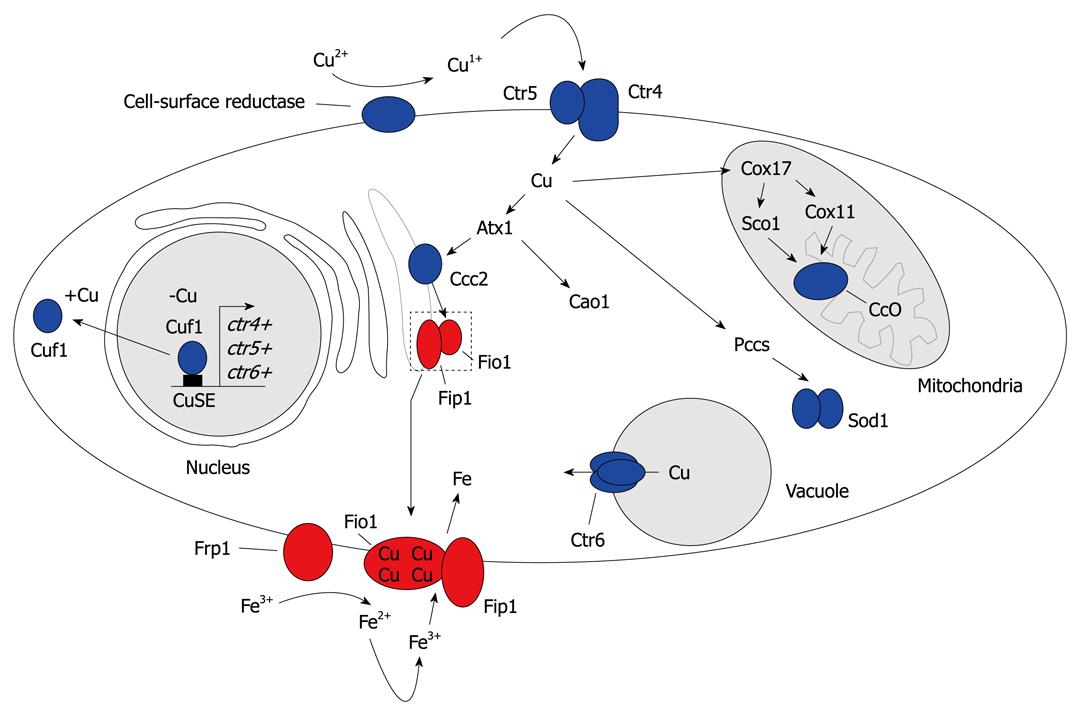
Figure 3 A model (steps 1-4) for copper homeostasis in Schizosaccharomyces pombe (Schiz.
pombe). Step 1: Copper is reduced from Cu2+ to Cu+ by a putative cell surface reductase. Following reduction, copper is transported across the plasma membrane by a heteroprotein complex formed by the Ctr4 and Ctr5 proteins; Step 2: Three copper chaperones, namely, Atx1, Pccs and Cox17, deliver copper to specific intracellular localizations. Atx1 shuttles copper from the cytosol to post-Golgi vesicles by docking specifically with the Ccc2 copper-transporting P-type ATPase. Once loaded, Ccc2 pumps copper into the lumen of the Golgi, which provides copper to the ferroxidase Fio1 as part of its maturation (with Fip1). Atx1 can function as a copper chaperone for a molecule other than Ccc2, which participates in the delivery of copper to Cao1. In the mitochondria, copper delivered by Cox17 is incorporated into cytochrome c oxidase, with the aid of Sco1 and Cox11. Pccs delivers copper to SOD1 in the cytosol; Step 3: When the pool of cytoplasmic copper is insufficient, Ctr6 is involved in copper efflux from the vacuole, which provides copper to cytosolic copper-dependent enzymes. Step 4: In response to copper deficiency, Cuf1 is localized in the nucleus where it activates the transport of copper by upregulating expression of the ctr4+, ctr5+ and ctr6+ genes. Conversely, in cells that undergo a shift from low to sufficient copper concentrations, Cuf1 translocates from the nucleus to the cytoplasm. The functional link between copper transport and iron uptake is explained by the observation that the Fip1 iron permease must couple with Fio1, which is a copper-dependent enzyme, to move through the secretory pathway and assemble as a functional iron-transport complex at the plasma membrane. Therefore, the inability to deliver copper to the interior of the cell, or to traffic copper appropriately to the lumen of the secretory compartment where it binds to Fio1, which leads to an iron-starvation defect.
- Citation: Labbé S. Simon Labbé’s work on iron and copper homeostasis. World J Biol Chem 2010; 1(5): 196-200
- URL: https://www.wjgnet.com/1949-8454/full/v1/i5/196.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i5.196