Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2010; 1(5): 109-132
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.109
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.109
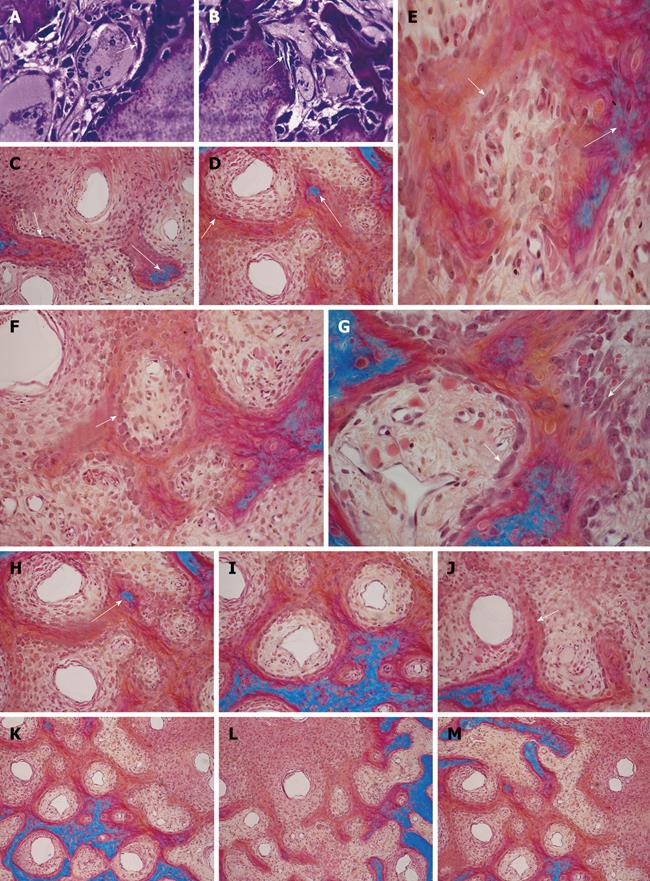
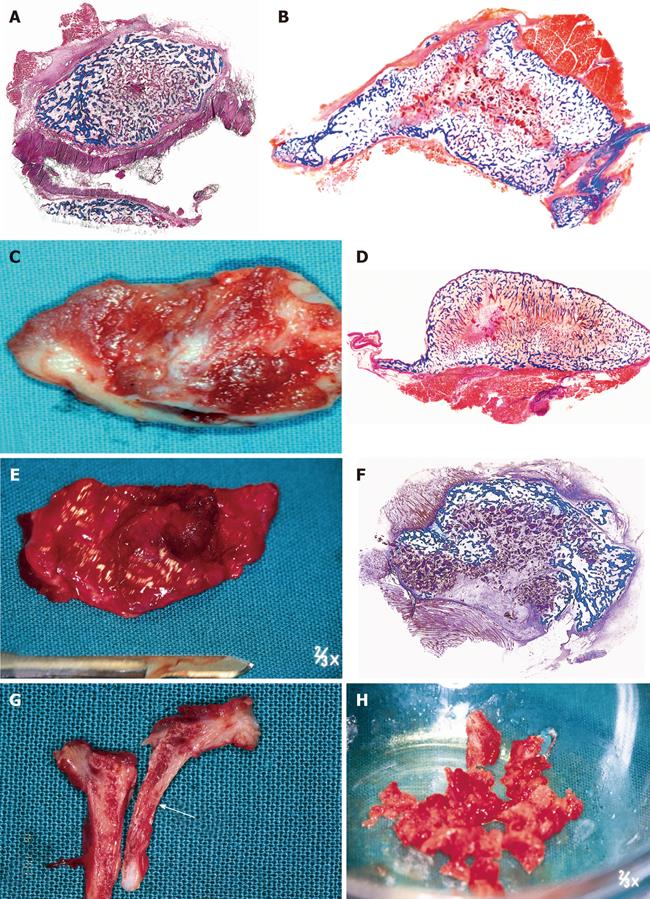
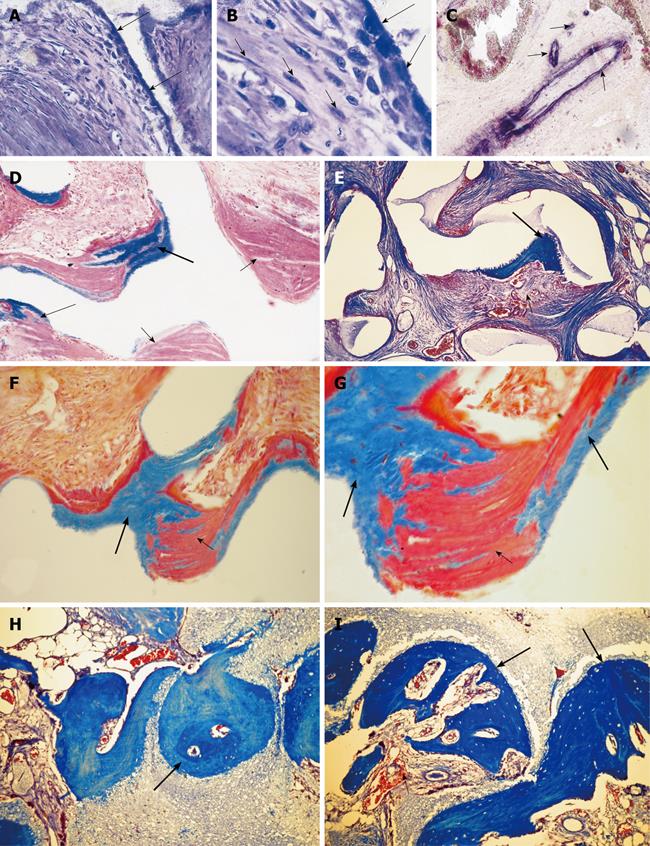
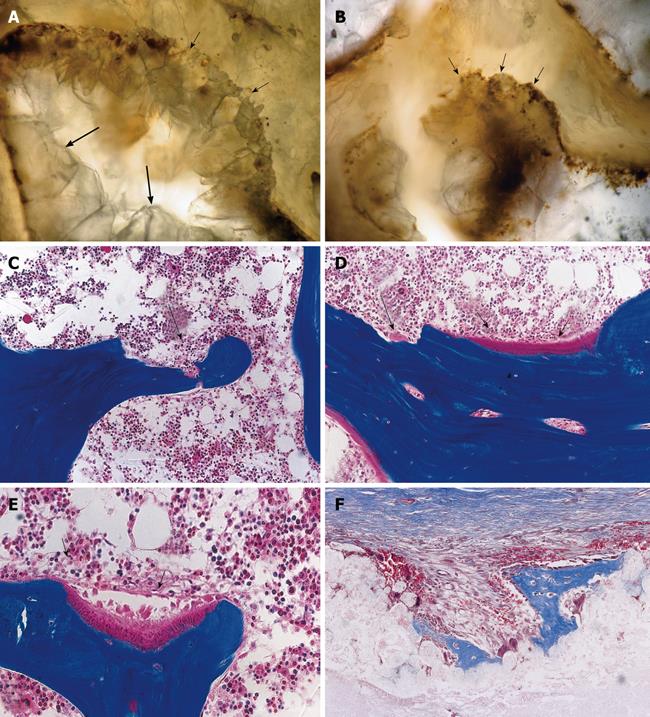
Figure 1 Three-dimensional angiogenesis, capillary sprouting, cellular trafficking and vascular and perivascular stem cell differentiation setting the induction of bone formation and the architecture of the cortico-cancellous osteonic bone.
A, B: Angiogenesis with exquisite intimate relationships between the invading capillaries and the collagenous matrix combined with 0.1-0.5 μg of osteogenin purified to apparent homogeneity from baboon bone matrices after subcutaneous implantation in rodents. Short arrows indicate vascular/perivascular cellular elements migrating from the vascular compartment to the bone forming compartment. The close proximity of the capillary to the newly differentiated osteoblastic cells seemingly provide a continuous flow of osteoprogenitor stem cells differentiating into bone forming cells as soon as pericytic cells move into the bone forming/osteoblastic compartment; C-M: Morphological stages of vascular tissue patterning and morphogenesis of the primate cortico-cancellous bone enveloping the central morphogenetic and osteogenetic vessels (short arrows in C and J). Long arrows point to foci of mineralization within the newly formed mesenchymal condensations with osteoblastic-like cells lining the morphogenetic condensation facing the central blood vessels (short arrow in F). Short arrows in D, E and G indicate osteoblastic-like cells. Mineralized mesenchymal highly cellular condensations (K, L, M) forming and remodeling around central morphogenetic and osteogenetic vessels pattern the induction of osteonic bone formation. Note the intimate relationships of the central blood vessels with osteoblastic/pre-osteoblastic-like cells and other stem cells surfacing the mesenchymal condensations patterning the newly formed osteonic cortico-cancellous bone. Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
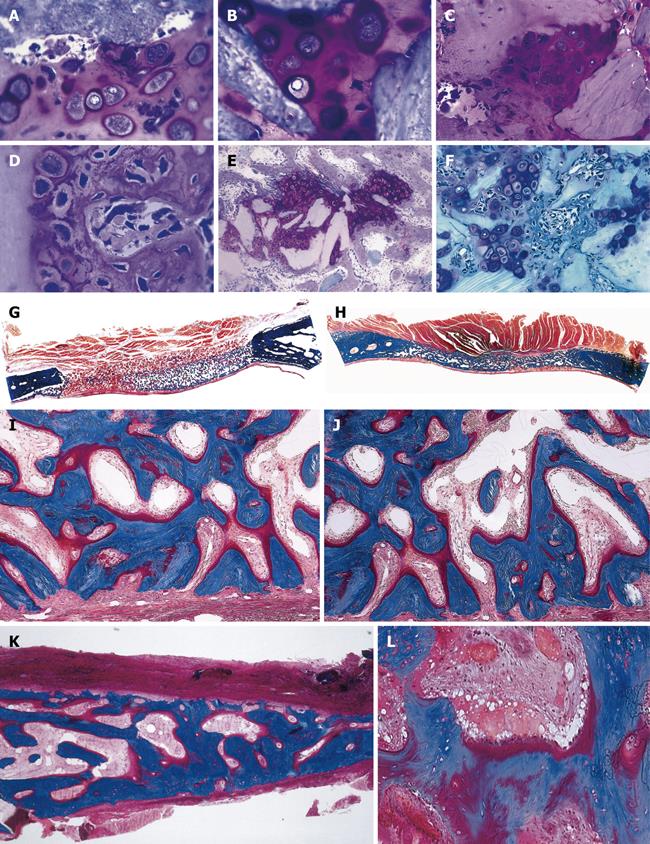
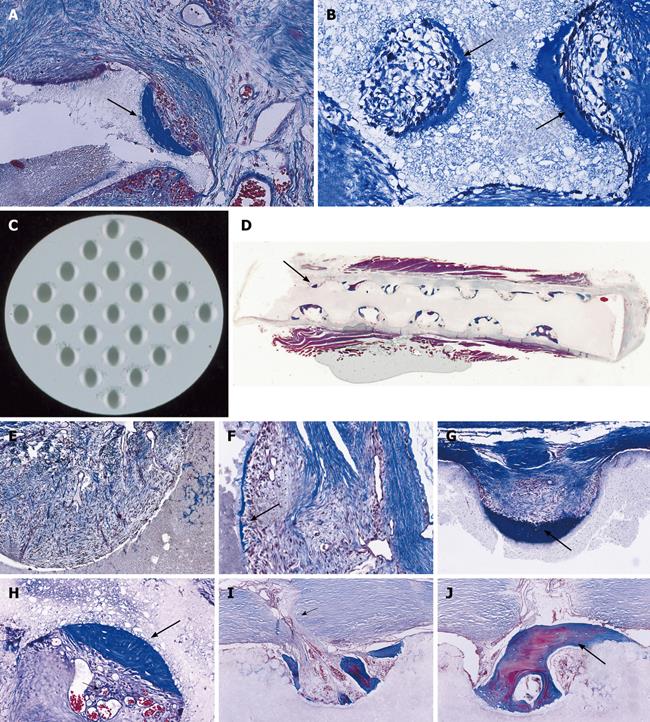
Figure 2 Tissue induction and morphogenesis upon recombination, or reconstitution, of extracted naturally-derived highly purified osteogenic soluble molecular signals with the insoluble signal of the inactive collagenous bone matrix in rodents and non-human primates of the species Papio ursinus (P.
ursinus). A-F: Differentiation of endochondral bone upon implantation of 0.1-0.5 μg osteogenin purified to apparent homogeneity reconstituted with rat insoluble and inactive collagenous bone matrix implanted subcutaneously in rats. Vascular invasion (D, F) initiates chondrolysis and osteoblastic-like cell differentiation by induction attached to the implanted collagenous matrix (F); G-J: Induction of bone formation on days 30 (G, I) and 90 (H, J) by naturally-derived highly purified osteogenic proteins extracted and purified from baboon bone matrices and implanted in non-healing calvarial defects of non-human primates P. ursinus. Mineralized bone (in blue) is surfaced by osteoid seams (red-orange) populated by contiguous osteoblasts; K, L: Highly purified bovine osteogenic proteins additionally purified by heparin-affinity chromatography column induce mineralized bone surfaced by osteoid seams 90 d after implantation in a massive mandibular defect of a human patient (K); high power view (L) shows the mineralized newly formed bone (in blue) surrounding the implanted collagenous matrix as carrier for the osteogenic proteins; this demonstrates the induction of bone formation in the human patient. A-F: Undecalcified section cut at 3 μm stained with toluidine blue after embedding in historesin; G-L: Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
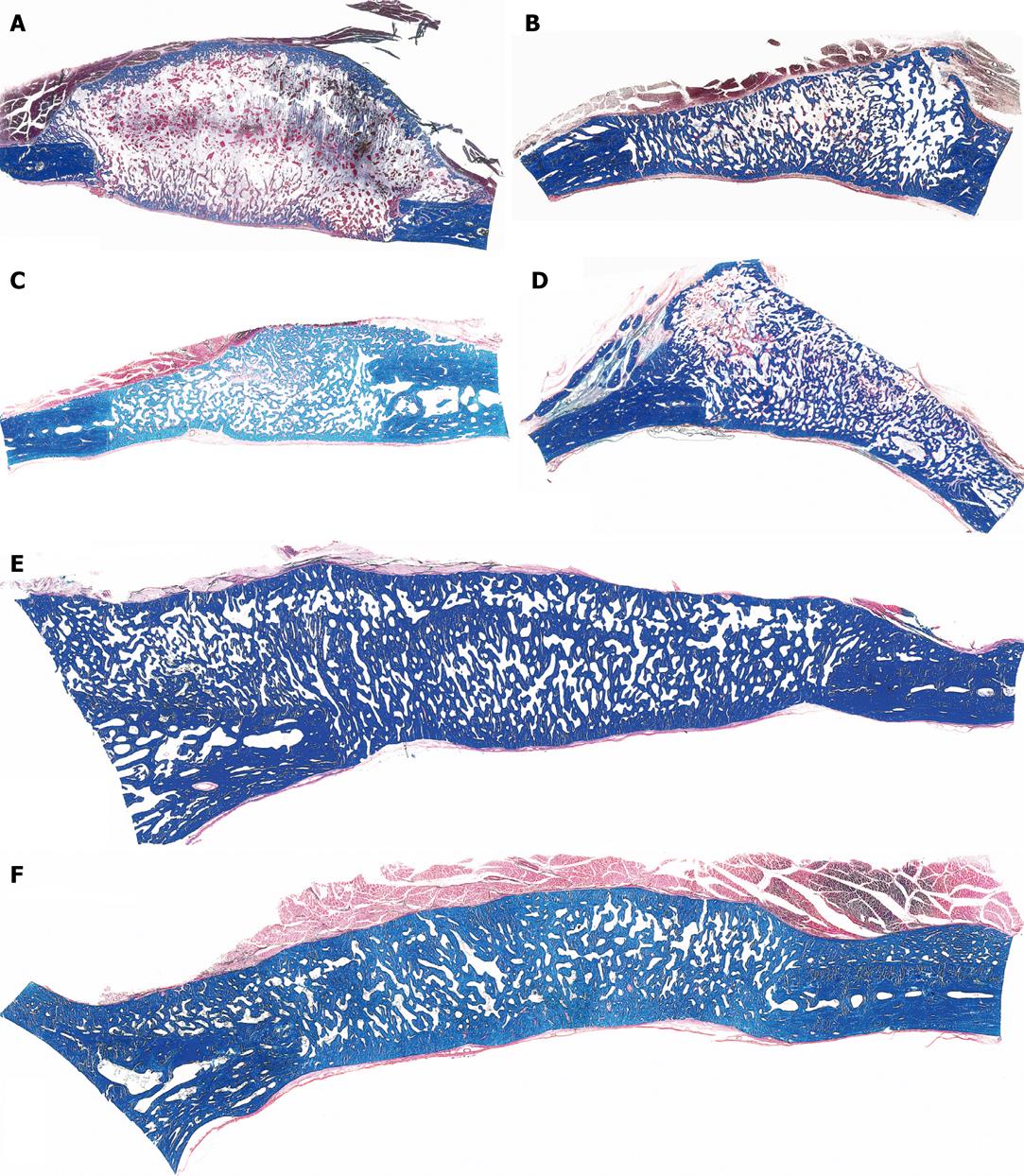
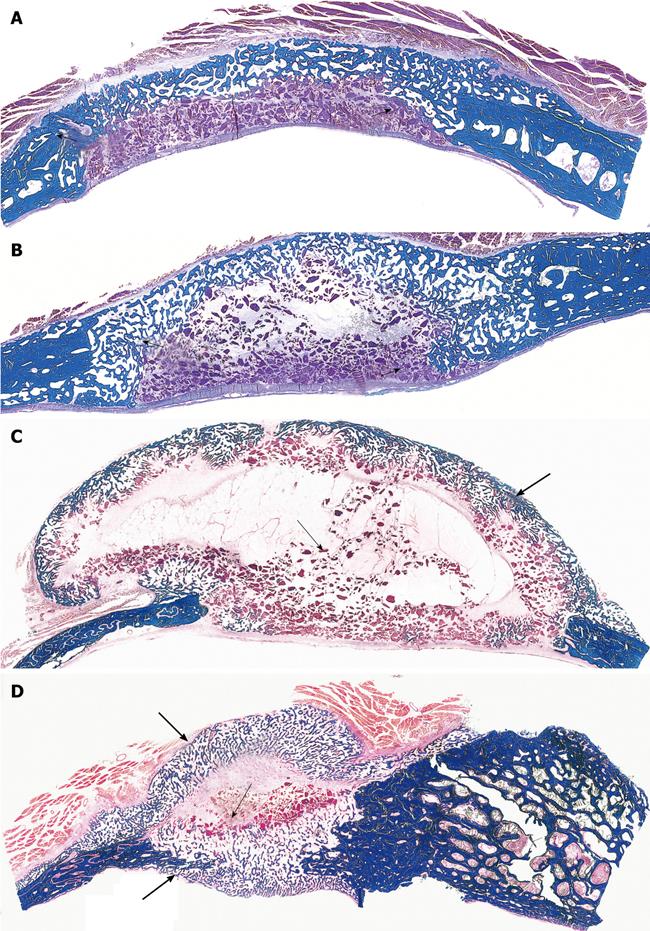
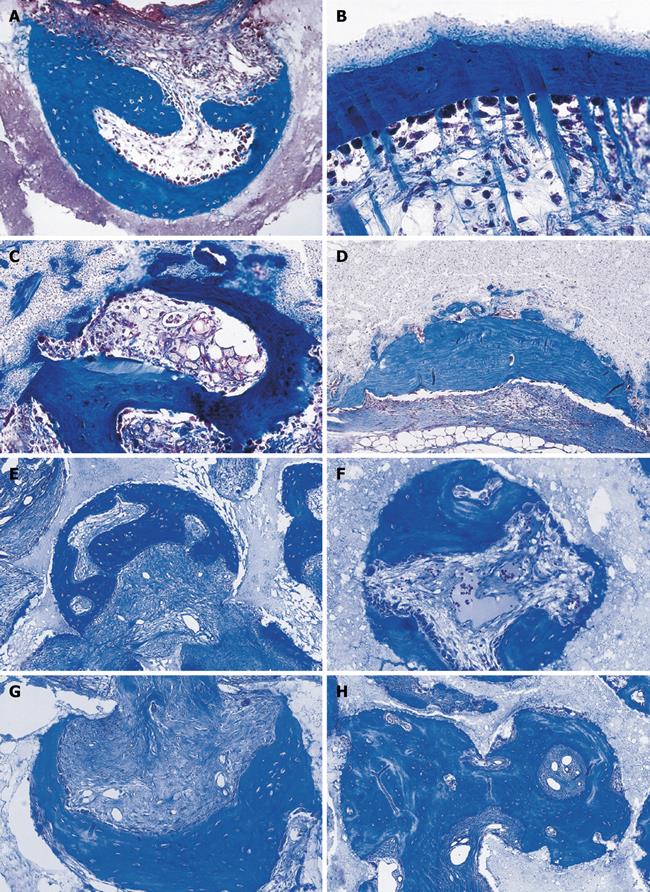
Figure 3 Calvarial tissue regeneration by doses of non-gamma irradiated recombinant human osteogenic protein-1 (hOP-1) implanted in non-healing calvarial defects of non-human primates P.
ursinus. A: Defect treated with 0.5 mg hOP-1 recombined with allogeneic insoluble collagenous bone matrix (ICBM) and harvested on day 30. Extensive mineralization and pronounced osteogenesis with displacement of the temporalis muscle overlying the implanted defect. Scattered remnants of the collagenous matrix as carrier embedded within a loose but highly vascular and cellular matrix; mineralized bone (in blue) facing the pericranial and endocranial aspect of the implanted hOP-1 osteogenic device; B: Remodeling and incorporation of the newly formed bone 90 d after implantation of 2.5 mg hOP-1 with corticalization of the endocranial aspect of the newly formed and mineralized bone; C, D: Exuberant induction of bone formation with peripheral corticalization of the newly formed bone 90 d after application of 0.1 and 2.5 mg hOP-1 per gram of allogeneic ICBM as carrier; E, F: Exuberant osteogenesis with solid block of remodeled bone particularly at the endocranial interface after implantation of 2.5 mg hOP-1 osteogenic devices harvested and processed for undecalcified histology on day 365 after implantation. A-F: Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
Figure 4 Calvarial tissue regeneration by doses of gamma-irradiated recombinant hOP-1 implanted in non-healing calvarial defects of non-human primates P.
ursinus. A: Heterotopic ossicle generated 30 d upon implantation of 0.5 mg hOP-1 in the rectus abdominis of P. ursinus showing prominent osteoid seams in orange red (long arrows) surfacing mineralized newly formed bone in blue (inset right panel of A); B-E: Tissue induction and morphogenesis by gamma-irradiated hOP-1 osteogenic devices implanted in calvarial defects and harvested on day 15 (B: 0.1 mg hOP-1), 30 (C, D: 0.5 mg hOP-1) and 90 (E: 0.5 mg hOP-1) after implantation, respectively. Note the time course of osteogenic tissue forming between the osteogenic pericranial and endocranial fronts with restitutio ad integrum 90 d after implantation; F, G: Solid cords of mineralized remodeled newly formed bone upon implantation of 2.5 mg gamma-irradiated hOP-1 in non-healing calvarial defects of P. ursinus. A-G: Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
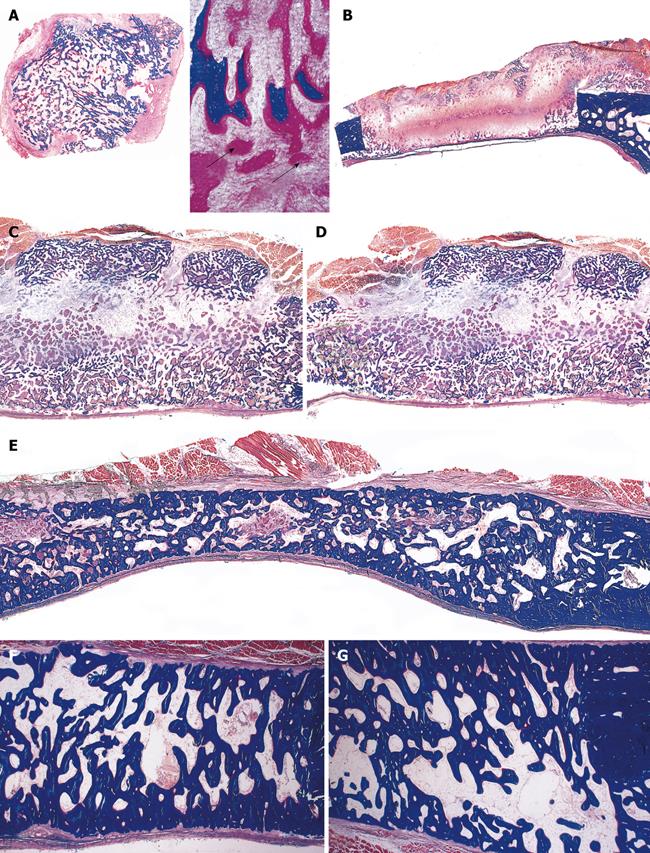
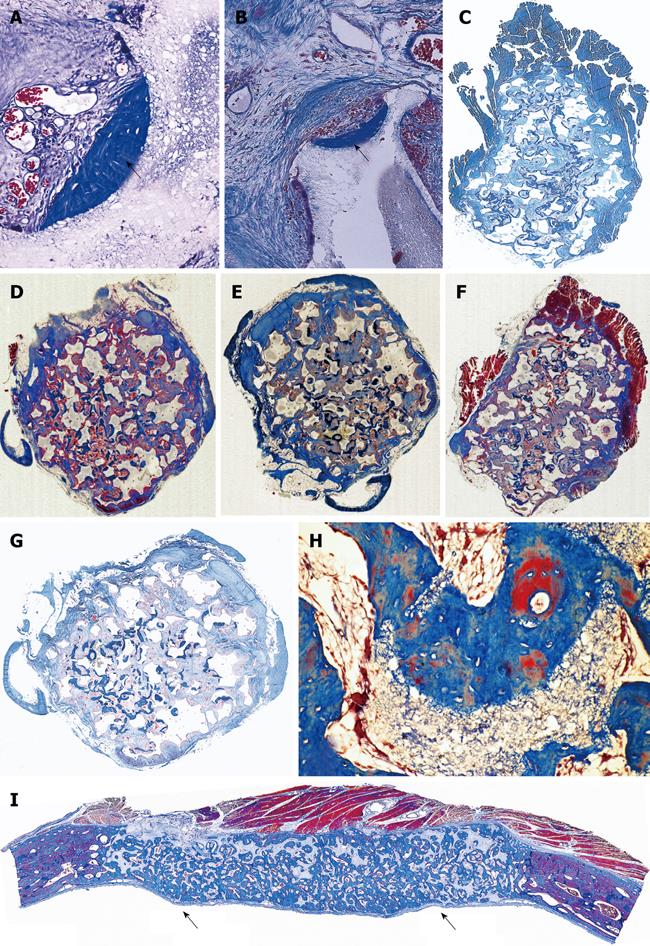
Figure 5 Apparent redundancy of molecular signals initiating the induction of bone formation in non-human primates P.
ursinus : heterotopic intramuscular bone induction by recombinant human transforming growth factor-β1 and -β3 (TGF-β1 and TGF-β3). A, B: Heterotopic induction of mineralized bone surfaced by osteoid seams after intramuscular implantation of 5 μg hTGF-β1 (A) and 125 μg hTGF-β3 (B) harvested on day 30; C, D: Large heterotopic ossicles induced by 125 μg hTGF-β3 and harvested on day 30 showing corticalization of the newly formed mineralized bone surrounding newly formed trabeculae of mineralized bone surfaced by large osteoid seams and scattered remnants of the implanted collagenous matrix as carrier; E: Heterotopic ossicle induced by 75 μg hTGF-β3 with substantial mineralization of the newly formed bone (blue in F) surrounding scattered remnants of collagenous matrix as carrier; G, H: Cut surfaces of mineralized and corticalized (arrow in G) ossicles induced by 75 μg hTGF-β3; Fragmented mineralized bone (H) can be used for autogenous transplantation in cranio-maxillo-facial defects. Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
Figure 6 Limited induction of bone formation by the recombinant hTGF-β3 and hTGF-β2 when implanted in calvarial defects of P.
ursinus but induction of massive ossicles after binary applications of recombinant hOP-1 with relatively low doses of platelet-derived pTGF-β1. A, B: Morphology of calvarial regeneration and induction of bone formation after application of 125 μg hTGF-β3 and 100 μg hTGF-β2, respectively, on day 90 after calvarial implantation. Note the induction of bone formation across the defects on the pericranial aspect only with limited if any bone formation at the endocranial dural aspect of both specimens. Short arrows point to the inhibition of bone formation within the fibrogenic collagenous matrix facing the newly formed bone originating pericranially and endocranially at the defect margins; C, D: Synergistic induction of bone formation upon implantation of binary application of 100 μg hOP-1 with 5 μg of porcine platelet-derived TGF-β1 (C) and 15 μg pTGF-β1 (D) 30 d after calvarial implantation. Prominent pericranial osteogenesis displacing the temporalis muscle; note the pericranial and endocranial osteogenetic fronts of mineralized newly formed bone (thick long arrows) surrounding scattered remnants of the collagenous matrix as carrier (thin long arrows). Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
Figure 7 Prominent induction of bone formation by binary applications of recombinant hOP-1 with relatively low doses of recombinant hTGF-β1 implanted in the rectus abdominis muscle of P.
ursinus. A, B: Morphology of tissue induction and rapid tissue growth by binary application of 25 μg hOP-1 and 0.5 μg hTGF-β1 harvested on day 15 after implantation in the rectus abdominis muscle. A: Induction of a corticalized ossicle on day 15 after the synergistic binary implantation in the rectus abdominis muscle; B: Trabeculae of mineralized bone in blue on day 15 are surfaced by osteoid seams (red-orange) populated by contiguous osteoblasts; C, D: Prominent induction of heterotopic bone after implantation of 25 μg hOP-1 contra laterally juxtaposed to doses of 5 μg[68] across the rectus abdominis muscle of adult non-human primates of the species P. ursinus[68]. The radius of activity of the singly implanted hTGF-β1 osteogenic devices synergistically enhanced the inductive activity of the contra laterally implanted 25 μg hOP-1 devices[68]; E, F: Large heterotopic mineralized and corticalized ossicles generated by binary application of 25 μg hOP-1 and 0.5 μg hTGF-β1 and harvested on day 30. Undecalcified sections cut at 6 μm stained free-floating with a modified Goldner’s trichrome.
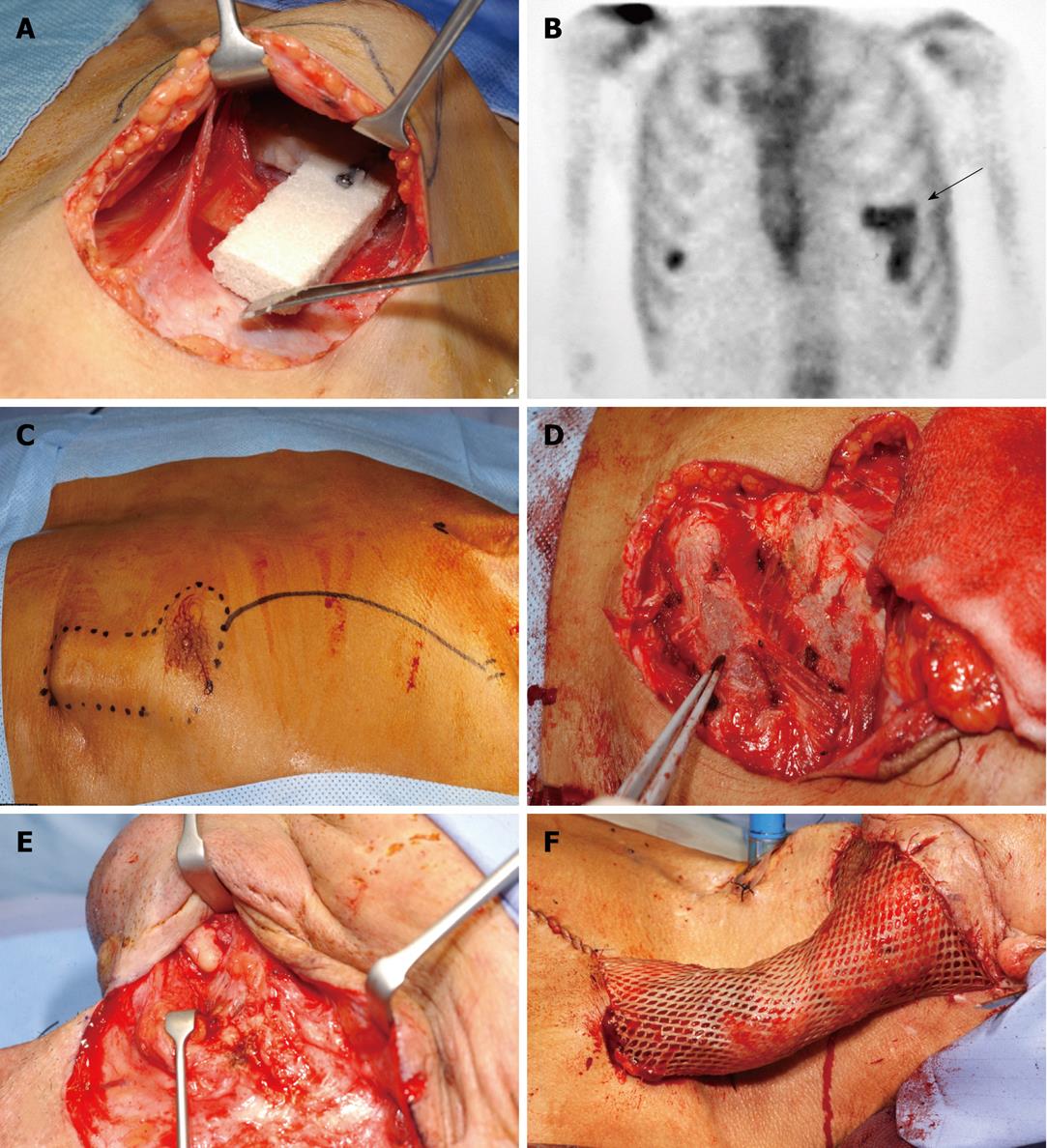
Figure 8 Prefabricated osteogenic protein-1/macroporous coral-derived hydroxyapatite implant pedicled into a vascularized bone flap transplanted into a mandibular defect of a human patient.
A: Surgical insertion of L-shaped blocks of coral-derived macroporous constructs into the muscular tissue of the chest of a human patient after ablative mandibular surgery for neoplastic mandibular lesion[79]. The implanted macroporous constructs were preloaded with recombinant hOP-1 before implantation into the left pectoralis major muscle; B: Skeletal scintigraphy demonstrates osteogenesis in the L-shaped pectoralis implant (arrow); C: Surgical debridement of the newly engineered construct; D, E: Preparation of the recipient mandibular bed; F: Placement and insertion of the pedicled flap into the previously operated left mandible[79]. Prefabricated bone flaps exploit the highly vascularized muscular tissue to generated osseous constructs for later transplantation in autogenous skeletal deficiencies.
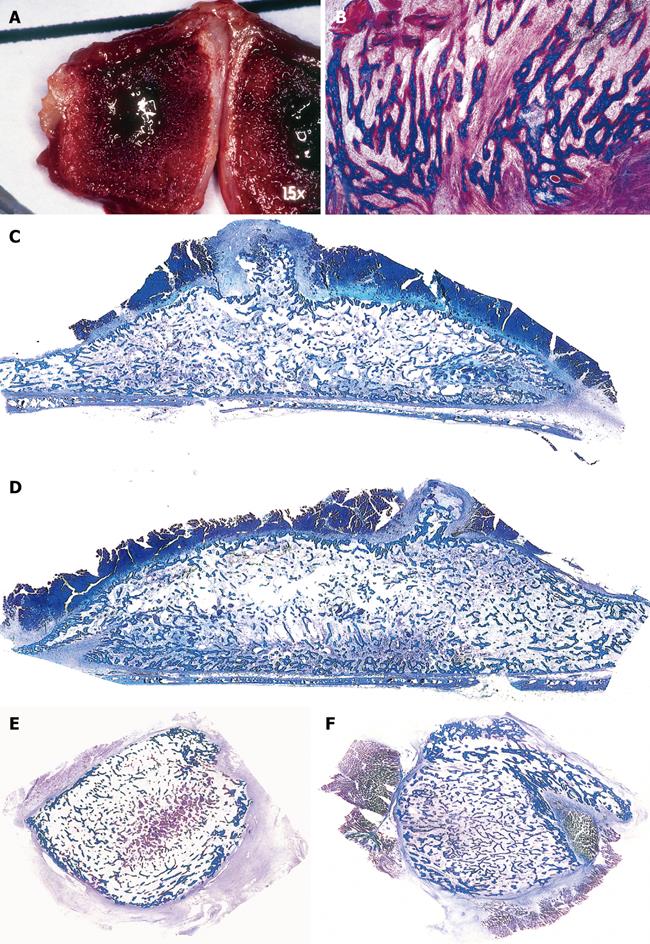
Figure 9 Intrinsic induction of bone formation by macroporous coral-derived hydroxyapatite matrices implanted in the rectus abdominis muscle of non-human primates of the species P.
ursinus. A-C: Morphology of cellular differentiation and vascular invasion by coral-derived hydroxyapatite constructs implanted in the rectus abdominis muscle. Differentiation of osteoblastic-like cells at the hydroxyapatite interface with hyper chromatic nuclei facing the highly vascularized stroma (long arrows); short arrows in B indicate a stream of locomoting perivascular cells leading to the hydroxyapatite surface for further differentiation and morphogenesis; C: Capillaries penetrating the macroporous spaces are osteogenetic in Trueta definition[9] having endothelial/perivascular cells intensely alkaline phosphatase positive cells; the osteogenetic vessel induces further capillary sprouting and invasion (short arrows) also highly positive for alkaline phosphatase; D: Undecalcified section showing mineralized cellular condensations in blue facing the substratum (thick and thin long arrows), short arrows point to collagenous condensations as yet to be mineralized; E: Using particulated granular coral-derived macroporous constructs, the morphogenesis of bone is only found within a concavity of the implanted biomimetic matrix, short arrow points to vascular invasion and angiogenesis, long arrow points to newly formed bone within the concavity. D and E were instrumental to the realization that the concavity is the geometric shape that induces the ripple-like cascade of bone differentiation by induction within the implanted macroporous constructs; F, G: Mineralization (thick long arrows) of mesenchymal collagenic condensations (short arrows) at the interface of the macroporous construct; H, I: Generation of substantial bone formation by induction in macroporous coral-derived biomatrices when implanted in the rectus abdominis and harvested on day 90. Arrows point to newly formed bone in blue within the concavities of the biomimetic matrices. Undecalcified and decalcified sections cut at 6 μm and stained with Goldner’s trichrome and toluidine blue.
Figure 10 Influence of geometry on bone induction and morphogenesis: self inducing geometric cues initiating the induction of bone without the exogenous application of the osteogenic proteins of the TGF-β supergene family.
A, B: Macroporous sintered crystalline hydroxyapatites and the spontaneous induction of bone formation within concavities (thick long arrows) of the heterotopically implanted substratum 30 d after implantation. Based on the digital images of Figure 9E, G and of the above digital microphotographs, solid discs of highly crystalline hydroxyapatites with a series of concavities on both planar surfaces (C) were constructed and implanted in the rectus abdominis muscle of adult non-human primates of the species P. ursinus; D: Histological analyses of the harvested discs show the reproducible induction of bone formation within the concavities of the substratum only (thick long arrow); bone initiates only within the pre-cut concavities of the biomimetic matrix; the images were responsible for the definition of the phenomenon of the “geometric induction of bone formation”[82,83]; E, F: Middle power views of vascular and mesenchymal tissue invasion on day 30 within concavities of highly crystalline sintered hydroxyapatites with capillary sprouting and the beginning of the induction of bone formation (arrow in F) attached to the implanted substratum; G: Thick long arrow point to newly induced bone on day 90 after implantation of the biomimetic matrix; H-J: Remodeling, growth and pattern formation of the newly formed bone within concavities of highly crystalline sintered hydroxyapatites with prominent vascular invasion (short arrows in H and I) feeding the newly formed and remodeled bone initiated within the concavities of the implanted substrata (thick long arrows in H and J). Decalcified sections cut at 6 μm and stained with Goldner’s trichrome.
Figure 11 Self inducing geometric cues: the concavity and the induction of bone differentiation by carbon-impregnated single-phase hydroxyapatite biomatrices and biphasic hydroxyapatite/tricalcium phosphate biomimetic matrices.
A, B: Prominent osteogenesis within carved concavities of carbon-impregnated single phase hydroxyapatite biomatrices 90 d after heterotopic implantation; B: Induction of bone formation by fine carbon-impregnated single phase hydroxyapatite with collagenic compact fibers protruding between osteoblastic cells into the fibrovascular space of the concavity; C: Induction of bone formation along a concavity of coarse carbon-impregnated hydroxyapatite scaffold inducing bone also in the bulk of the sintered construct; D: Maintenance and remodeling of the newly formed bone 180 d after heterotopic implantation of carbon-impregnated single-phase hydroxyapatite coarse biomatrix; E-H: Induction of bone in macroporous spaces with concavities initiating and maintaining the induction of bone formation 90 and 180 d after intramuscular implantation. Decalcified sections cut at 6 μm and stained with Goldner’s trichrome.
Figure 12 Bone induction regulated by a series of repetitive concavities assembled within the macroporous spaces of highly crystalline sintered hydroxyapatite implanted in the rectus abdominis of non-human primates P.
ursinus. A, B: Bone (short arrows) with associated vascular invasion initiates within concavities of heterotopically implanted substrata. Devices were implanted in the rectus abdominis muscle without the addition of osteogenic proteins of the TGF-β supergene family; C-G: Macroporous sintered calcium phosphate constructs harvested from the rectus abdominis on day 90: low power views of five specimens harvested from different animals showing the reproducible intrinsic induction of bone formation by the geometric motif of the concavity highlighted in (H); I: Low power view of the prominent induction of bone formation (short arrows) across the macroporous spaces of a sintered construct on day 90 previously implanted in a calvarial defect. Decalcified sections cut at 6 μm and stained with Goldner’s trichrome.
Figure 13 Biomimetism of the remodeling cortico-cancellous unit of the primate osteonic bone with the induction of bone formation by the concavities assembled in macroporous calcium phosphate-based biomimetic matrices.
A, B: Remodeling cycles of osteoclastic activity with pits, lacunae and concavities (short arrows) cut by osteoclastogenesis as seen in fossilized skeletal remains of Australopithecus africanus, the man-ape of Southern Africa[8]. Long arrows indicate crystal of calcium phosphate accumulated during fossilization in highly calcareous and wet environments (Grounded/polished sections by van den Heever B); C-E: Osteoclastogenesis cut pits and lacunae ultimately in the form of concavities in extant P. ursinus species (long arrows). Concavities cut by osteoclastogenesis are then filled with newly formed bone (formation phase of the remodeling cycle) with significant synthesis of osteoid matrix (D, E), short arrows in D and E point to newly formed osteoid seams within the concavities after osteoclastogenesis; F: Limited resorption/dissolution of a biphasic hydroxyapatite/tricalcium phosphate matrix initiates the induction of bone formation. C-E: Undecalcified sections cut at 6 μm stained with Goldner’s trichrome; F: Decalcified section cut at 6 μm.
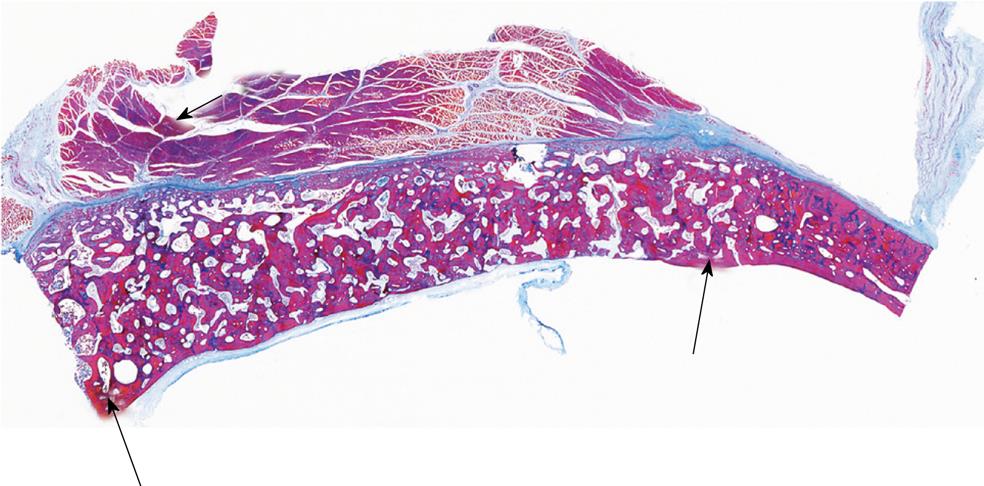
Figure 14 Complete regeneration of a calvarial defect in P.
ursinus 365 d after implantation of a macroporous construct of hydroxyapatite/β-tricalcium phosphate. Long term incorporation of the biphasic biomimetic matrix within the calvarium showing restitutio ad integrum of the calvarial defect; long arrows indicate the defect margins showing bone formation by induction across the macroporous spaces with the newly generated bone higher than the recipient calvarium; short arrow indicates the overlying temporalis muscle. Decalcified section cut at 6 μm.
- Citation: Ripamonti U. Soluble and insoluble signals sculpt osteogenesis in angiogenesis. World J Biol Chem 2010; 1(5): 109-132
- URL: https://www.wjgnet.com/1949-8454/full/v1/i5/109.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i5.109