Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Dec 26, 2010; 1(12): 369-376
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.369
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.369
Figure 1 Suofu Qin, PhD, Senior Scientist, Retinal Disease Research, Department of Biological Sciences, Allergan, Inc.
, 2525 Dupont Drive, Irvine, CA 92612-1599, United States.
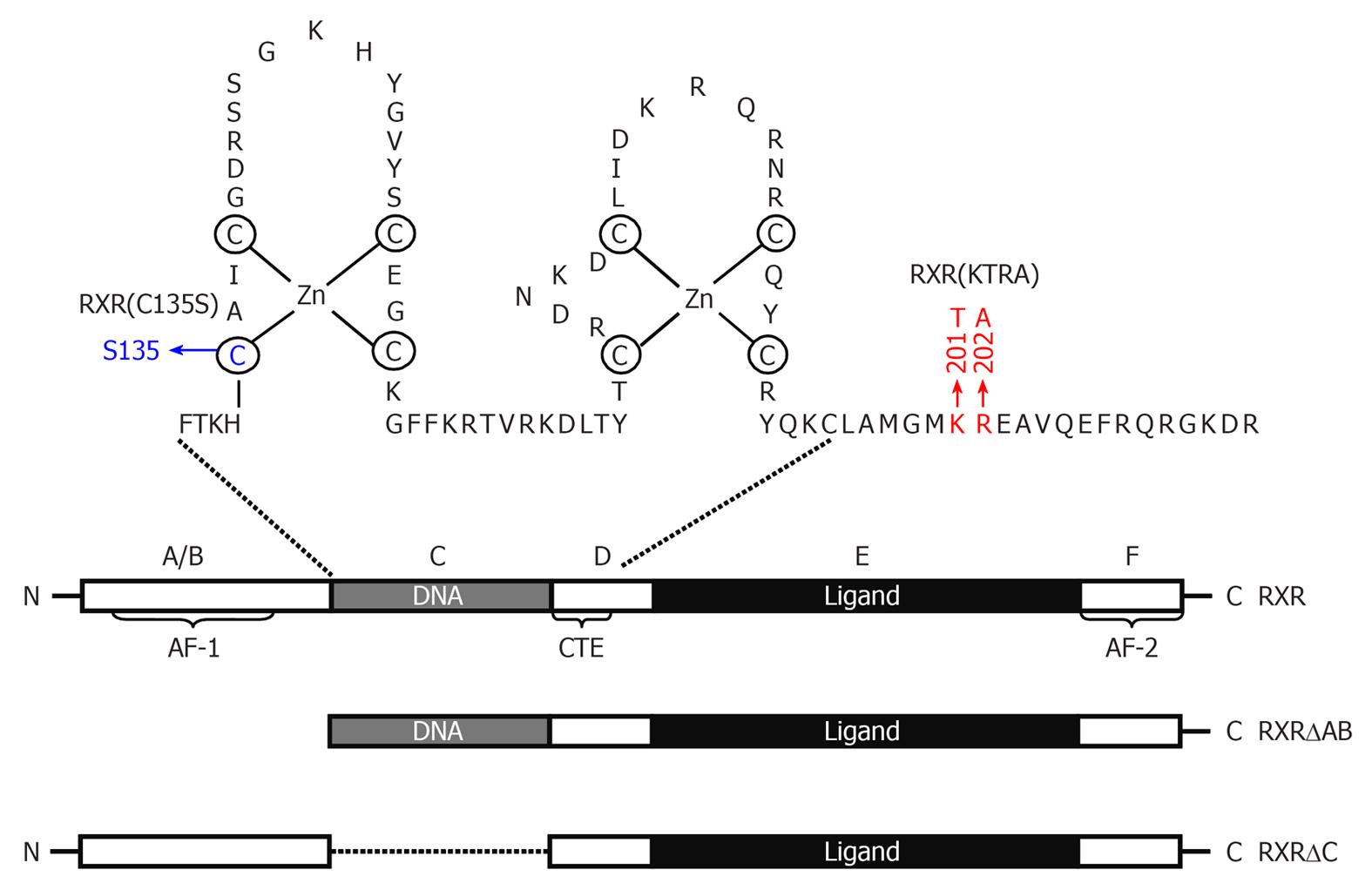
Figure 2 Schematic representation of Retinoid X receptor α protein and its deletion mutants.
Retinoid X receptor (RXR)α consists of a variable N-terminal domain (A/B domain) containing activation function 1, a highly conserved DNA-binding domain (DBD), also named as C domain, a nonconserved hinge (D domain), a moderately conserved ligand-binding domain (E domain), and a ligand-dependent transactivation F domain (AF-2). The DBD consists of two cysteine-rich zinc-finger motifs through which nuclear receptors bind to a specific DNA sequence and a carboxy-terminal extension (CTE) that exists only in RXRs and RXR-binding partners. Point mutation of Cys135 to Ser in first zinc-finger disables the capability of RXR to bind DNA. KTRA mutations in the CTE disrupts RXRα homodimerization but does not affect RXRα heterodimerization. Ligand-binding domain contains a ligand-binding pocket for binding of small, lipophilic molecules, a cofactor binding surface, and a dimerization surface.
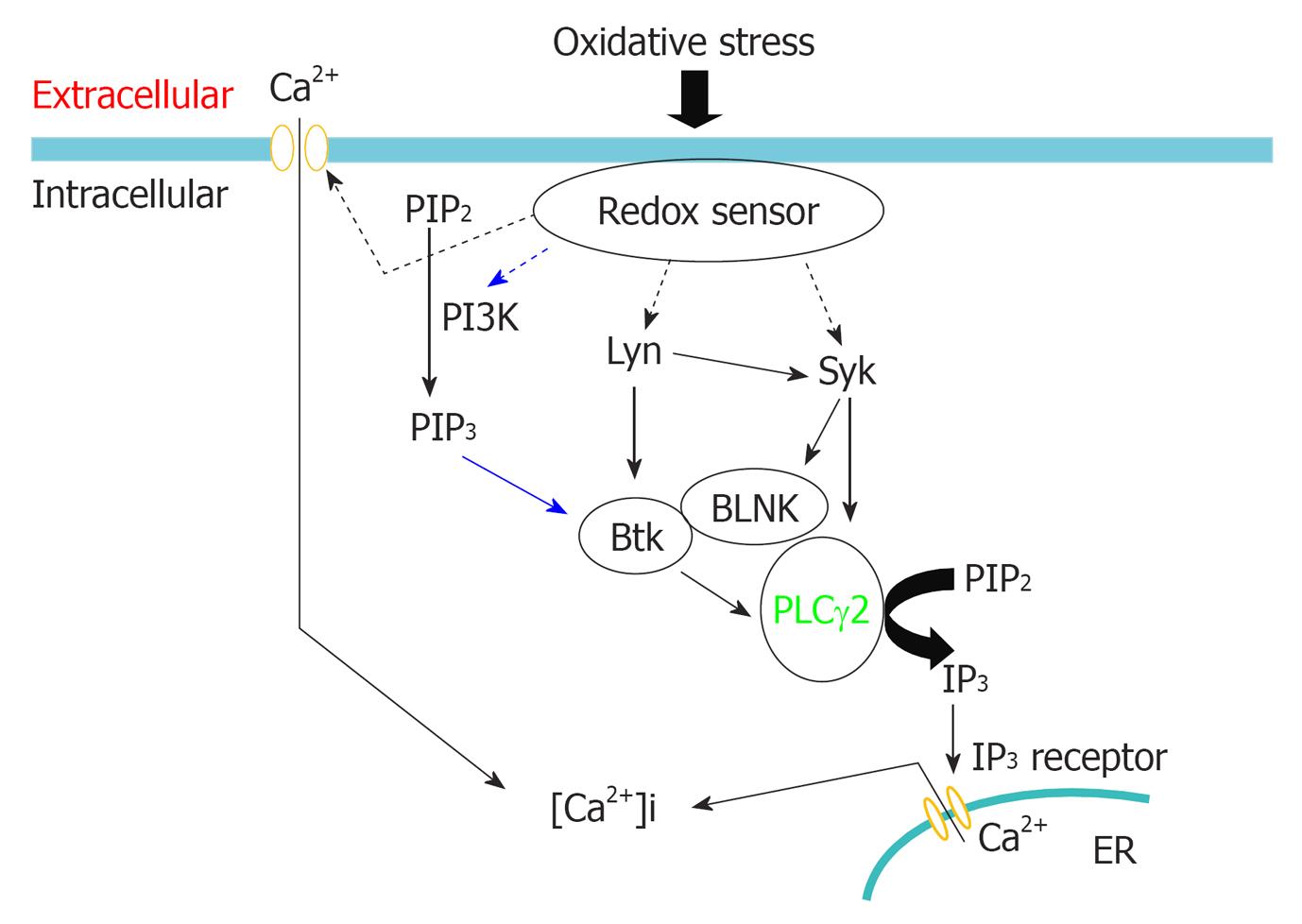
Figure 3 Proposed model for Syk in mediating oxidative stress-induced calcium release.
Phosphorylation of Syk by Lyn and autophosphorylation is required for oxidative stress-induced activation of Syk. How Lyn senses oxidative stress in DT40 cells is currently unknown. Oxidative stress might inhibit tyrosine phosphatase, thereby activating Lyn; Activated Syk phosphorylates phospholipase Cγ2 (PLCγ2) and adaptor protein B cell linker protein (BLNK). Phosphorylated BLNK provides docking sites for PLCγ2 and Btk, enable Btk phosphorylates PLCγ2. Simultaneous phosphorylation of PLCγ2 by Syk and Btk is indispensable for oxidative stress-induced activation of PLCγ2; Activated PLCγ2 cleaves phosphatidylinositol 4,5-biphosphate, releases IP3, triggering Ca2+ release via binding to ryanodine receptor. Oxidative stress also activates PI3K pathway signaling through recruitment of p85 PI3K to the particulate fraction, producing phosphatidylinositol 3,4,5-trisphosphate that targets the activated PLCγ2 to its substrate site for maximal catalytic efficiency.
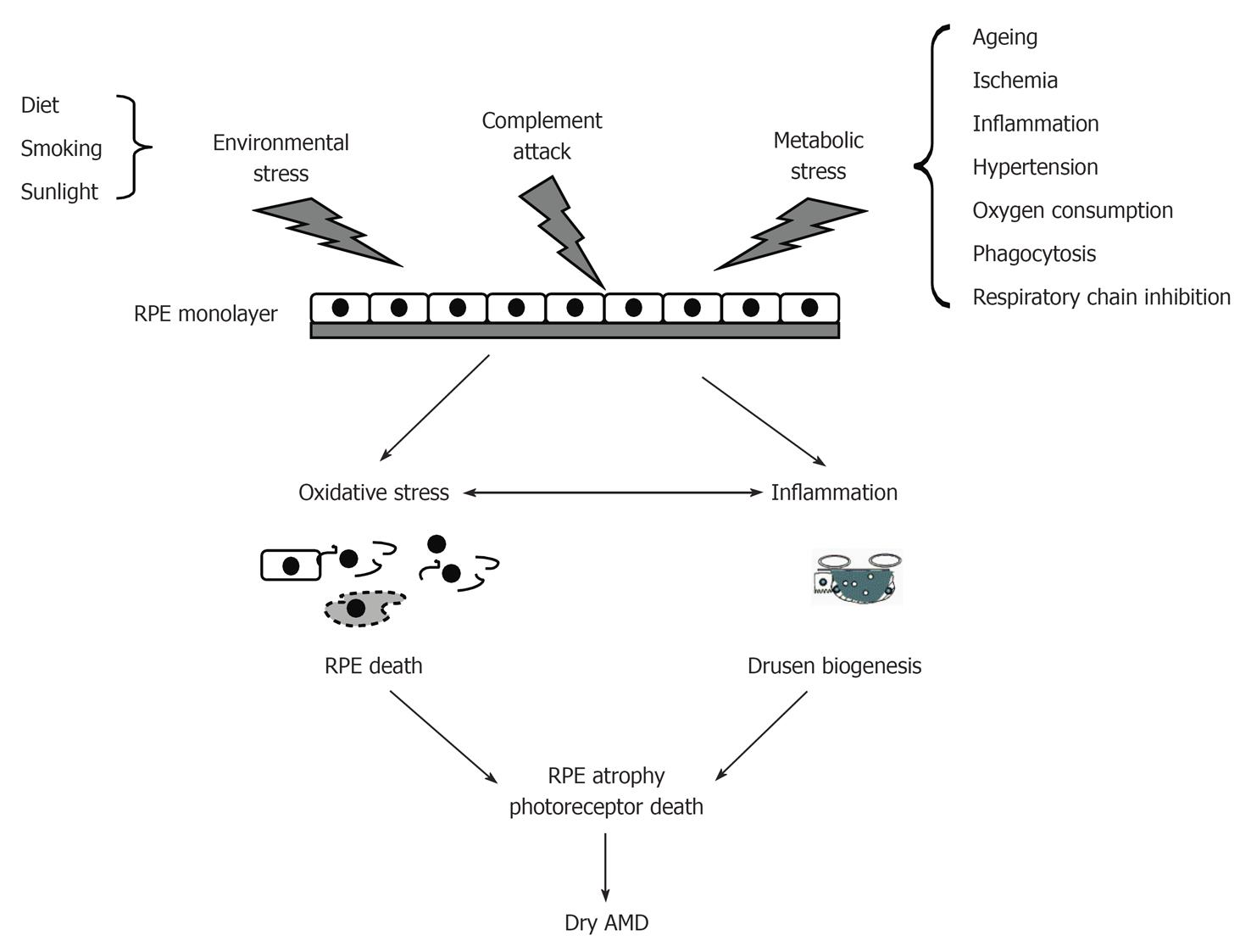
Figure 4 Conditions that contribute oxidative stress and inflammatory injury of retinal pigment epithelial cells and subsequent etiology of dry age-related macular degeneration.
Retinal pigment epithelial (RPE) cells can be injured by environmental stress, complement attack, and metabolic stress. Injured RPE cells undergo cell death and inflammatory-related drusen biogenesis, leading to atrophy of RPE cells. AMD: Age-related macular degeneration.
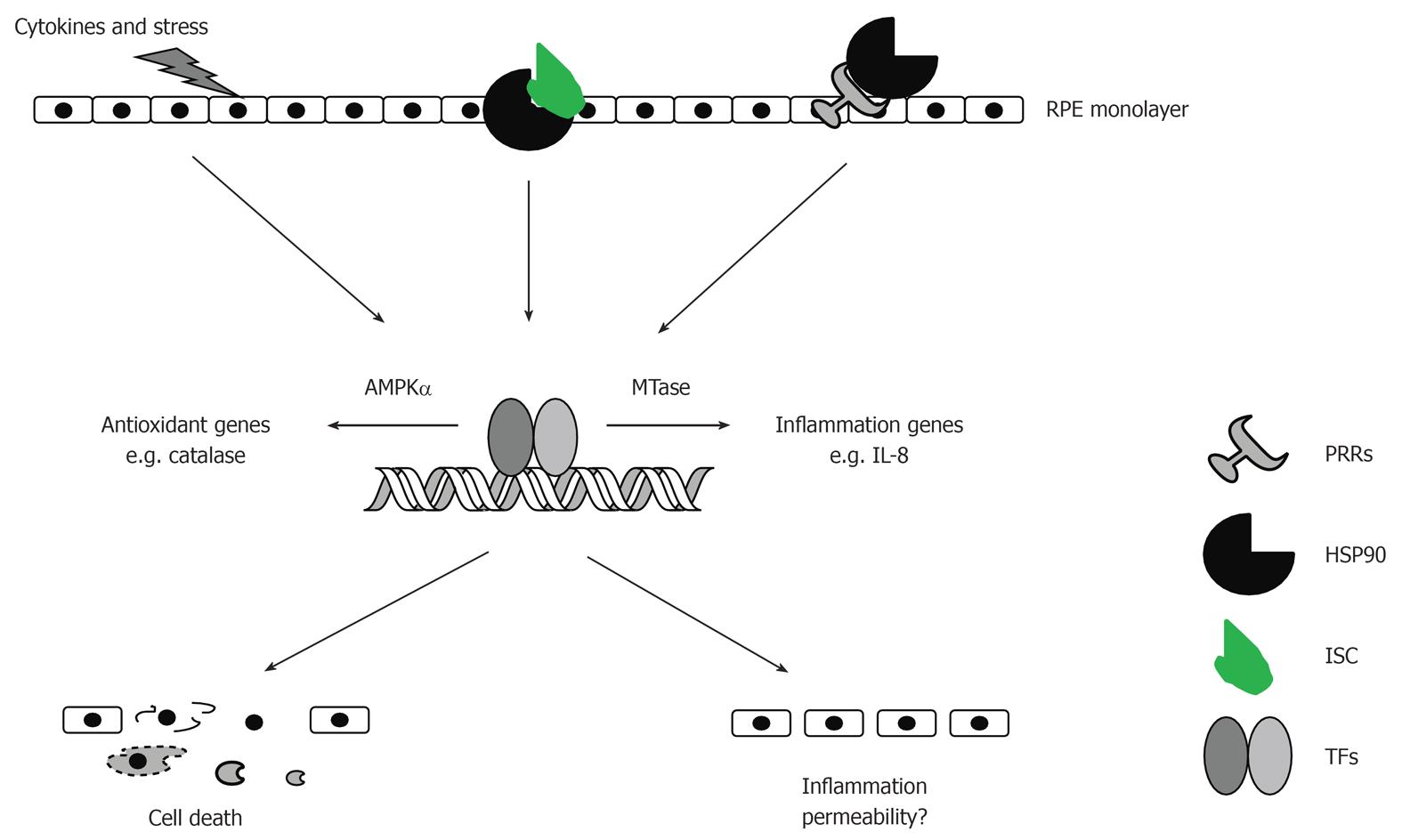
Figure 5 Potential roles of AMP-activated protein kinase, heat-shock protein-90, interleukin-8, and MTases in the development of retinal diseases.
AMP-activated protein kinase (AMPK)α1 controls expression of antioxidant genes while AMPKα2 maintains assembly of tight junctions in retinal pigment epithelial (RPE) cells. Heat-shock protein-90 (HSP90) can function as a danger signal once released from injured cells or as a holding chaperone for assembly of inflammatory signaling complex. Interleukin (IL)-8 causes RPE cell injury via an autocrine feedback activity. MTases promote transcription of inflammation genes through methylation of proteins and DNA. MTases: S-adenosylmethionine-methyltransferases; PRRs: Pattern-recognition receptors; ISC: Inflammatory signaling components; TFs: Transcription factors.
- Citation: Qin S. Suofu Qin’s work on studies of cell survival signaling in cancer and epithelial cells. World J Biol Chem 2010; 1(12): 369-376
- URL: https://www.wjgnet.com/1949-8454/full/v1/i12/369.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i12.369