Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Dec 26, 2010; 1(12): 353-361
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.353
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.353
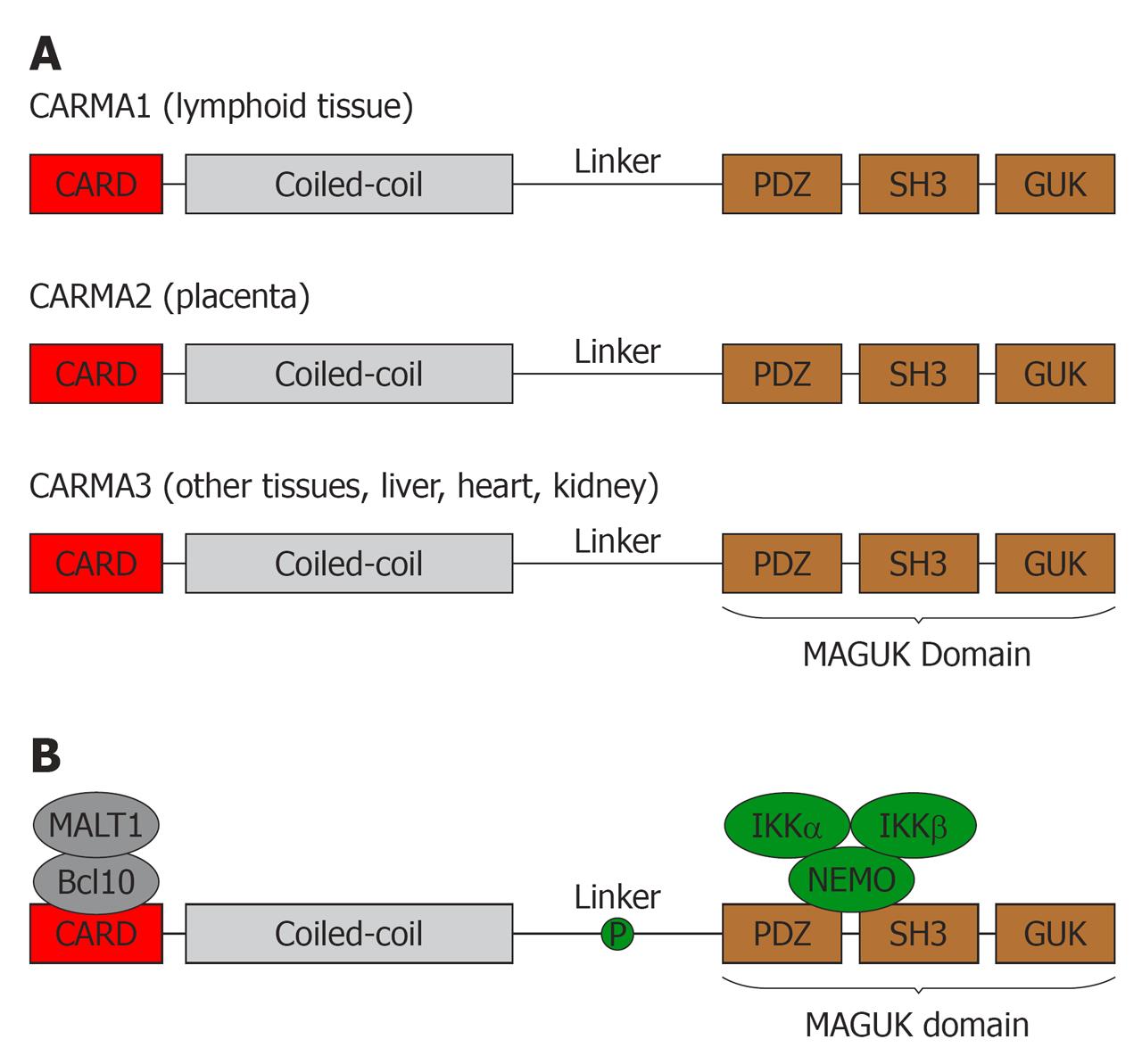
Figure 1 CARD recruited membrane associated protein family members.
A: The CARD recruited membrane associated (CARMA) protein family has three members: CARMA1, CARMA2 and CARMA3. Each member shares similar structures: an N-terminal caspase-recruitment domain (CARD), followed by a coiled-coil domain (CC), a linker region, a PDZ domain, an SH3 domain, and a GUK-like domain. Although all CARMA protein members share similar structures, they are transcribed by distinct genes, and expressed in different tissues. Specifically, CARMA1 is predominantly expressed in spleen, thymus, and peripheral blood leukocytes; CARMA2 is expressed only in placenta; and CARMA3 is expressed in a broad range of tissues, especially highly in liver, kidney, heart, and brain, but not in spleen, thymus, or peripheral blood lymphocytes; B: Upon activation, the linker region is phosphorylated. The CARD domain of CARMA protein interacts with Bcl10, which further binds MALT1, while PDZ and SH3 domains associate with the IκB kinase complex via NF-κB essential modulator (NEMO). Additionally, different CARMA proteins also interact with other unique signaling molecules. For example, CARMA3 interacts with β-arrestin 2, whereas CARMA1 associates with ADAP.
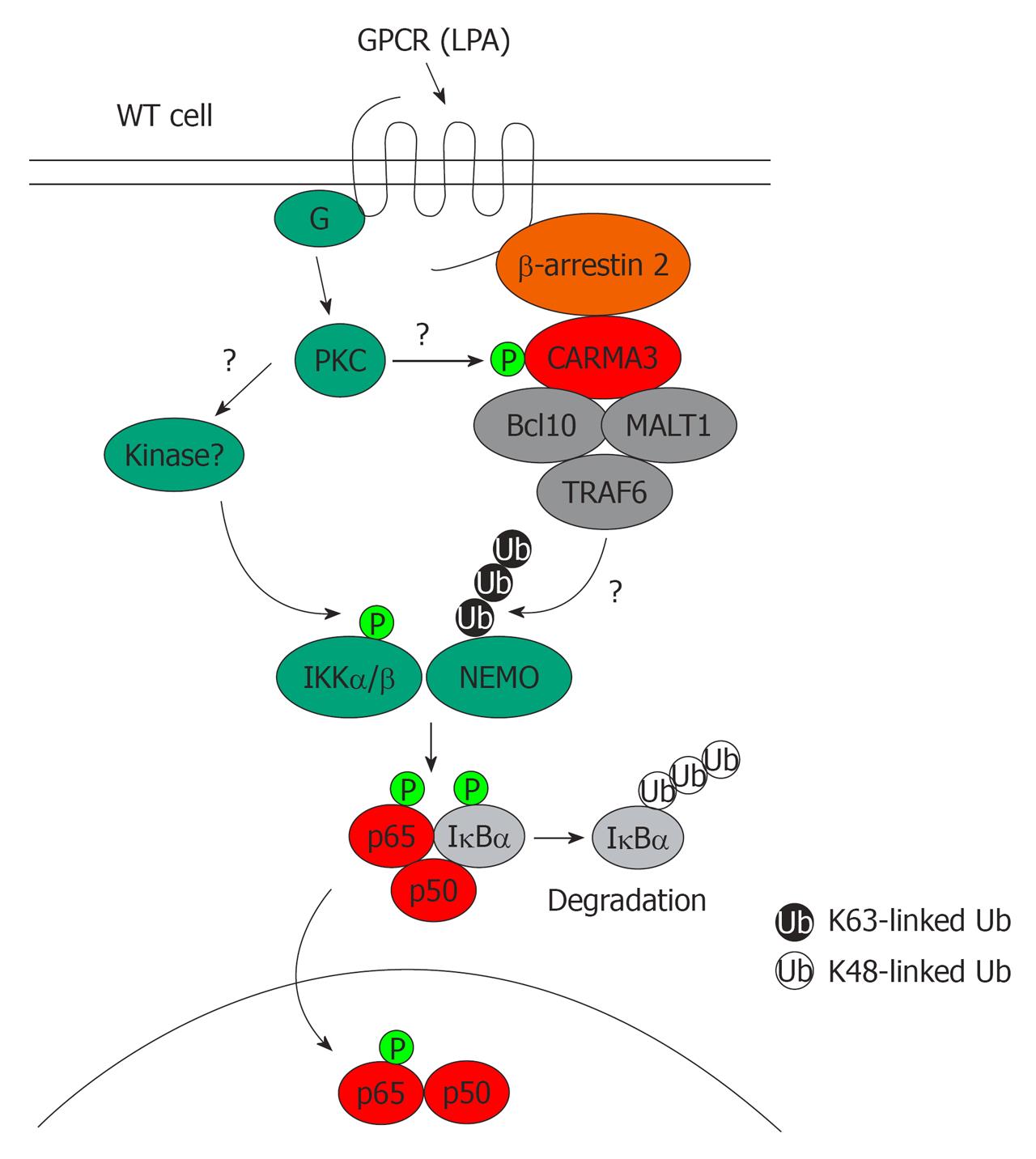
Figure 2 Working model of CARD recruited membrane associated protein 3-dependent nuclear factor-κB activation in the G protein-coupled receptor (lysophosphatidic acid) signaling pathways.
G protein-coupled receptor (GPCR) [lysophosphatidic acid (LPA)]-induced nuclear factor (NF)-κB activation involves the recruitment of CARD recruited membrane associated protein 3 (CARMA3) to the receptor by β-arrestin 2, which leads to formation of the CARMA3/Bcl10/MALT1/TRAF6 complex, which results in polyubiquitination of the IκB kinase (IKK) complex. A CARMA3-independent, PKC-dependent signal induces phosphorylation of the IKK complex by an unknown kinase in the presence of GPCR. After IKK is both polyubiquitinated [NF-κB essential modulator (NEMO)] and phosphorylated (IKK), it is activated, which leads to NF-κB activation. In the absence of CARMA3, GPCR (LPA)-induced polyubiquitination of the IKK complex is defective, which results in defects in IKK and NF-κB activation. WT: Wild-type; Ub: Ubiquitin.
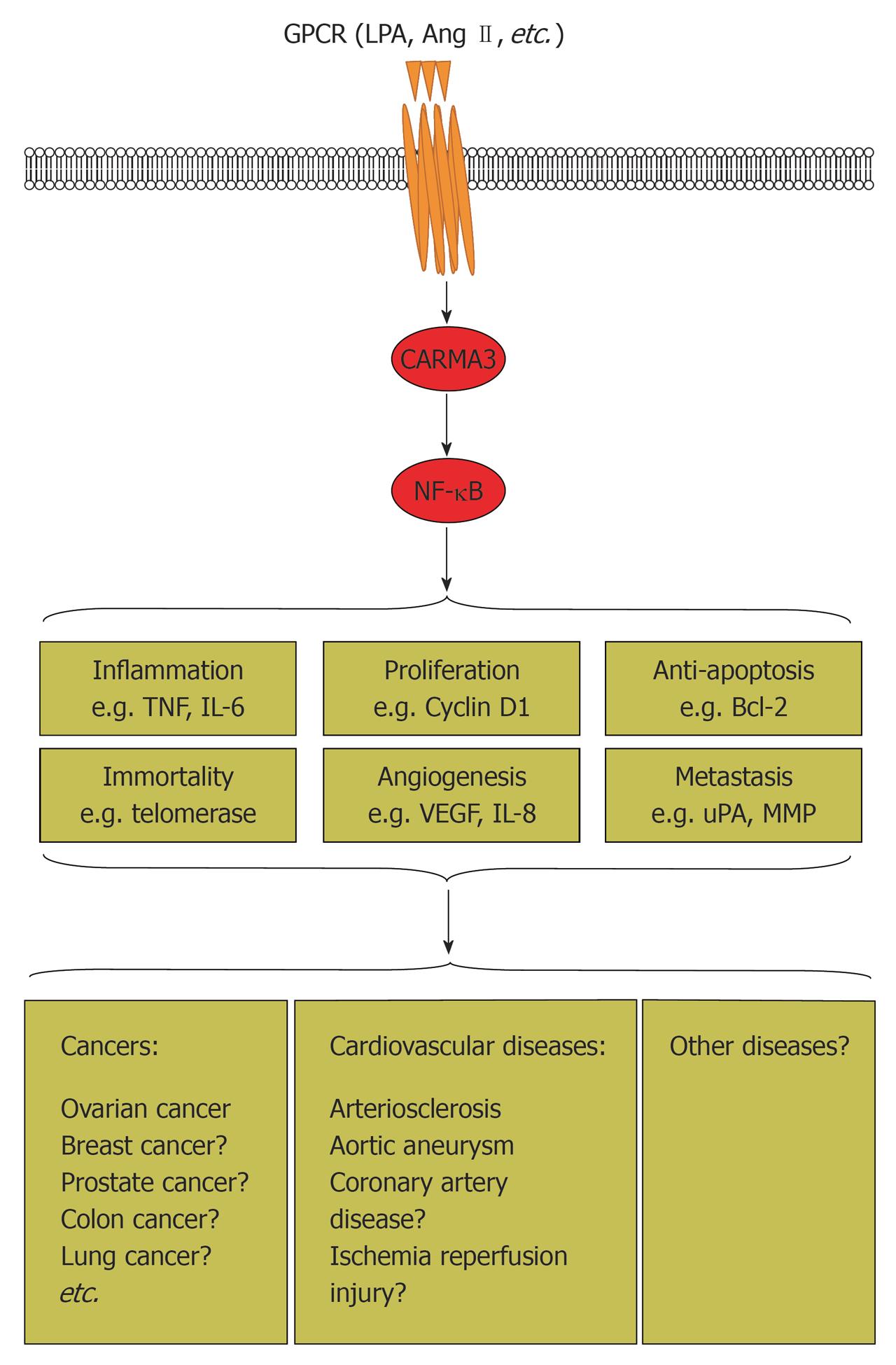
Figure 3 Proposed working model of G protein-coupled receptor/CARD recruited membrane associated protein 3/nuclear factor-κB signaling pathways in cancer, cardiovascular diseases, and other diseases.
G protein-coupled receptor (GPCR) [lysophosphatidic acid (LPA), angiotensin (Ang) II] activates CARD recruited membrane associated protein 3 (CARMA3), which in turn triggers nuclear factor (NF)-κB activation. NF-κB plays an important role in regulation of many physiological and pathological processes. Aberrant regulation of the GPCR/CARMA3/NF-κB signaling axis results in cancer, cardiovascular diseases, and probably other diseases. Mechanistically, it promotes cell proliferation, angiogenesis and metastasis, and inhibits apoptosis. In addition, it also induces inflammation. CARMA3 is indispensable for GPCR (LPA, Ang II)-induced NF-κB activation. Consequently, it plays pivotal roles in GPCR-induced tumor progression and cardiovascular diseases. Full definition of GPCR/CARMA3/NF-κB signaling events could aid the discovery of new drug targets and production of profoundly significant clinical therapies for cancer, cardiovascular diseases, and many other diseases. TNF: Tumor necrosis factor; IL: Interleukin; VEGF: Vascular endothelial growth factor; uPA: Urokinase plasminogen activator; MMPs: Matrix metalloproteinases.
- Citation: Sun J. CARMA3: A novel scaffold protein in regulation of NF-κB activation and diseases. World J Biol Chem 2010; 1(12): 353-361
- URL: https://www.wjgnet.com/1949-8454/full/v1/i12/353.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i12.353