Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Oct 26, 2010; 1(10): 298-306
Published online Oct 26, 2010. doi: 10.4331/wjbc.v1.i10.298
Published online Oct 26, 2010. doi: 10.4331/wjbc.v1.i10.298
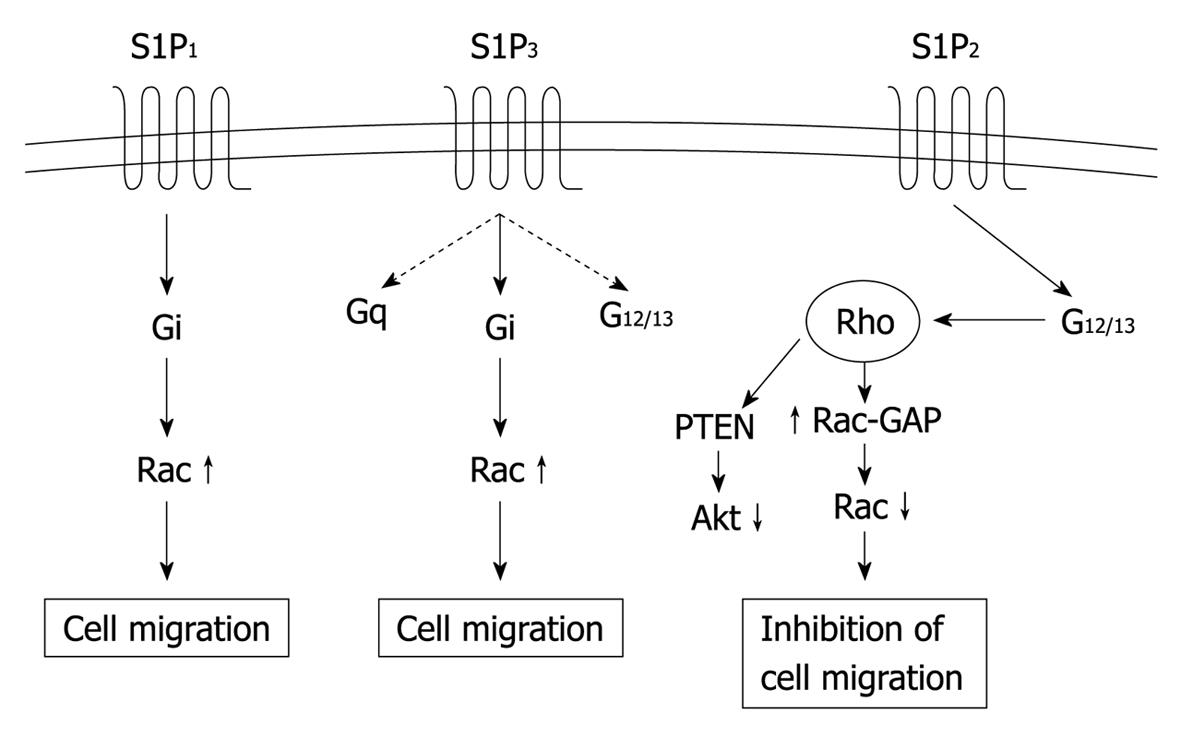
Figure 1 Receptor-subtype-specific signaling mechanisms of S1P1, S1P2 and S1P3.
These three receptors activate partially overlapping but distinct sets of signaling pathways via coupling to different sets of the heterotrimeric G proteins. S1P1 and S1P3 induce activation of Rac in a Gi-dependent manner to stimulate cell migration, whereas S1P2 inhibits Rac via G12/13-Rho to mediate inhibition of cell migration. S1P2 also stimulates 3’-specific polyphosphoinositide phosphatase “phosphatase and tensin homolog deleted from chromosome 10” (PTEN) to inhibit the serine/threonine protein kinase Akt, which probably participates in inhibition of cell migration. S1P: Sphingosine-1-phosphate.
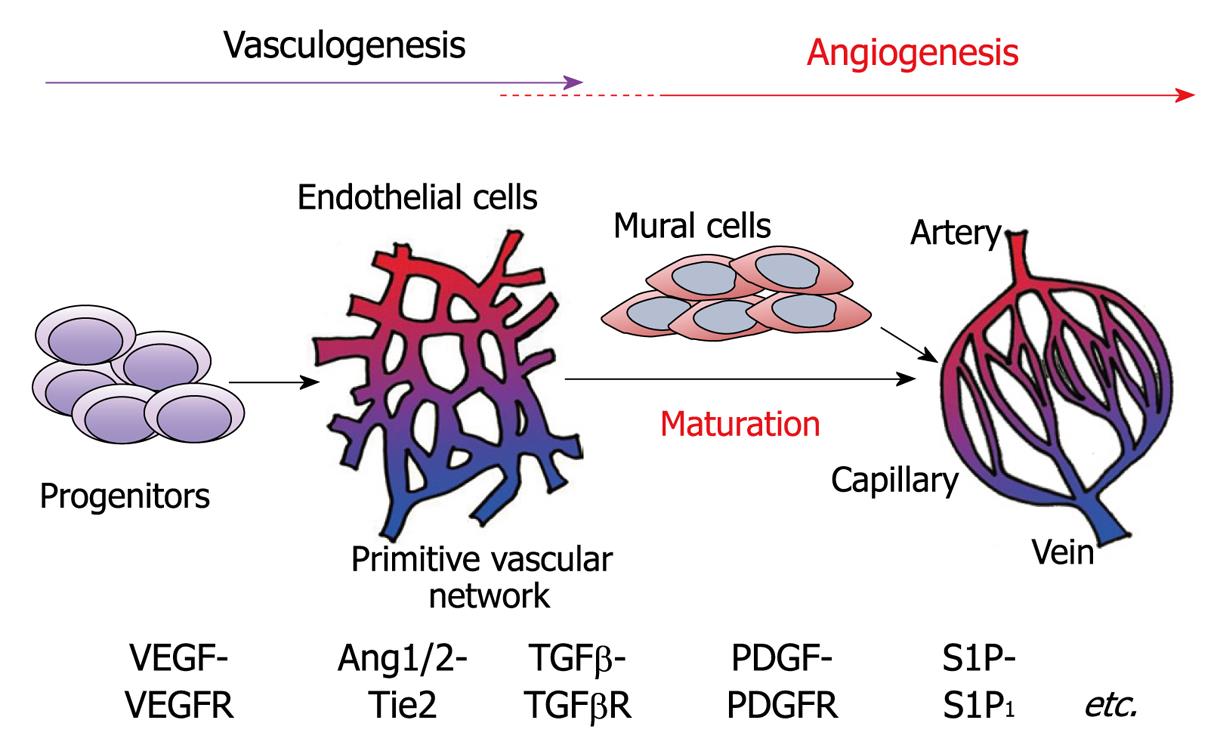
Figure 2 Schematic representation of vascular formation.
The processes include vasculogenesis and angiogenesis. During vasculogenesis and angiogenesis, various growth factors and their receptors play spaciotemporally specific roles. Newly formed vessels are stabilized by the recruitment of mural cells, smooth muscle cells and pericytes. S1P: Sphingosine-1-phosphate; VEGF: Vascular endothelial growth factor; TGF: Transforming growth factor; PDGF: Plateletderived growth factor; VEGFR: VEGF receptor; TGFβR: TGFβ receptor; PDGFR: PDGF receptor.
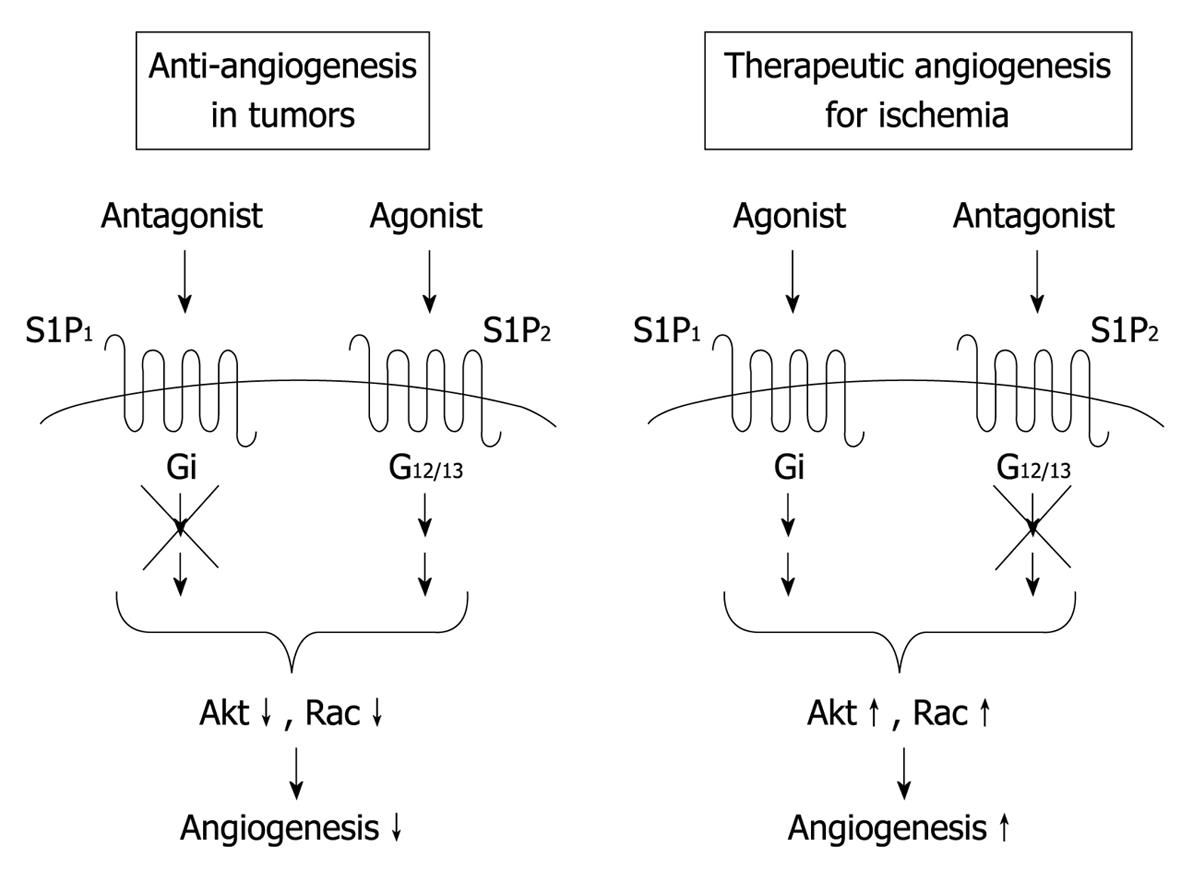
Figure 3 Possible therapeutic strategies for anti-angiogenesis in tumors and angiogenesis in ischemic diseases.
S1P1 and S1P2 mediate angiogenic and anti-angiogenic effects of S1P in vivo, respectively. Therefore, it is possible that simultaneous activation of the angiogenic receptor S1P1 and blockade of the anti-angiogenic receptor S1P2 are more effective for therapeutic angiogenesis in ischemic diseases compared with S1P1 activation or S1P2 inhibition alone. In contrast, for anti-angiogenic therapy in cancer, the combination of S1P1 inhibition and S1P2 stimulation might be favorable.
- Citation: Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N, Yoshioka K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J Biol Chem 2010; 1(10): 298-306
- URL: https://www.wjgnet.com/1949-8454/full/v1/i10/298.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i10.298