Published online Sep 27, 2017. doi: 10.4240/wjgs.v9.i9.193
Peer-review started: February 8, 2017
First decision: March 9, 2017
Revised: August 23, 2017
Accepted: September 3, 2017
Article in press: September 4, 2017
Published online: September 27, 2017
Processing time: 241 Days and 13 Hours
To evaluate the feasibility of a text-messaging system to remotely monitor and support patients after discharge following elective colorectal surgery, within an enhanced recovery protocol.
Florence (FLO) is a National Health Service telehealth solution utilised for monitoring chronic health conditions, such as hypertension, using text-messaging. New algorithms were designed to monitor the well-being, basic physiological observations and any patient-reported symptoms, and provide support messages to patients undergoing colorectal surgery within an enhanced recovery after surgery protocol for 30 d after discharge. All interactions with FLO and physiological readings were recorded and patients were invited to provide feedback.
Over a four-week period, 16 out of 17 patients used the FLO telehealth service at home. These patients did not receive telephone follow-up at three days, as per our standard protocol, unless they reported being unwell or did not make use of the technology. Three patients were readmitted within 30 d, and two of these were identified as being unwell by FLO prior to readmission. No adverse events attributable to the use of the technology were encountered.
The utilisation of telehealth in the early follow-up of patients who have undergone major colorectal surgery after discharge is feasible. The use of this technology may assist in the early recognition and management of complications after discharge.
Core tip: Remote follow-up in the immediate post-discharge period utilising telehealth is feasible, and may help identify patients at risk of developing complications sooner, leading to earlier proactive management.
- Citation: Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B, Lobo DN, Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: A feasibility study. World J Gastrointest Surg 2017; 9(9): 193-199
- URL: https://www.wjgnet.com/1948-9366/full/v9/i9/193.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i9.193
Unplanned readmissions to hospital in the United Kingdom increased by 52% between 1992-1999 and 2007-2008[1]. The National Health Service (NHS) faces a predicted disparity between resources and patient need of nearly 30 billion by 2020-2021[2]. Introduced in 2011, the financial penalties (Payment by Results) apportioned to NHS Hospital Trusts for patients readmitted within 30 d of discharge have created concern for health care providers, who face the challenge of balancing timely discharge against the risk of early readmission.
In 2013, a telephone follow-up call was provided to patients between two and four days following discharge for patients undergoing colorectal surgery as part of an enhanced recovery after surgery (ERAS) protocol at Nottingham University Hospitals (NUH)[3]. The telephone call is designed to provide emotional and psychological support to patients following discharge, but also to identify and address any symptoms and to reiterate advice about successful recovery[3].
A number of telehealth solutions are now available for healthcare providers, including telephone follow up[3], text messaging[4,5], mobile applications[6], video conferencing[7], and automated device transmission[8]. FLO, short for “Florence”, is an NHS telehealth solution (in collaboration with Mediaburst Ltd., Manchester, United Kingdom) that has been shown to be effective in helping to manage hypertension[4], and is an acceptable modality of healthcare provision for patients[7]. The aim of this feasibility study was to investigate FLO in the early follow-up of patients who have been discharged from hospital after colorectal surgery within an ERAS protocol, and to assess patients’ perceptions of this modality of short-term follow-up.
This evaluation was conducted at an 1100-bedded United Kingdom teaching hospital, where around 380 major colorectal procedures are performed each year within an ERAS protocol. Target lengths of stay for patients on ERAS pathways for laparoscopic and open procedures are 3 and 5 d, respectively[9].
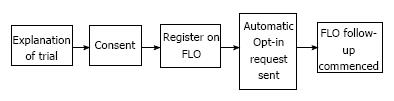
This service evaluation was conducted over a four-week period. Patients were identified at their pre-operative assessment, and provided with a brief explanation and an information leaflet about the trial. Following surgery, patients were approached 24-48 h prior to their predicted discharge date, by either the ERAS nurse or ERAS fellow, and a more detailed explanation of the service was offered (Figure 1). Those who opted in to use FLO were followed-up remotely by FLO every day for 30 d after discharge.
At any stage during the follow-up period, the patient would be able to text in the word “stop” and the service would terminate. Patients who declined to participate, and those who were ineligible, or failed to opt-in or utilise the telehealth service having opted in, received a telephone call from the ERAS nurse practitioner between 2 and 4 d following discharge, as per the usual care[3]. Telephone follow-up was not performed if patients had reported being well to FLO for 4 d, but subsequently opted out of the service prior to completing 30 days’ follow-up.
Eligible participants were those who had undergone a colorectal procedure as part of an ERAS protocol, had mental capacity, were willing to participate, possessed a mobile phone and had experience with sending and receiving text messages. Patients who required reoperation or who were admitted to the intensive care or high dependency units were not invited to participate.
The Nottingham ethics committee deemed this study to be a service evaluation, and formal ethical committee review was not warranted. Informed, written consent was obtained from all patients.
The algorithms were designed utilising FLO editing software. These were based on the telephone algorithms currently used by the ERAS nurse practitioner, and were categorised as well-being checks, support messages, physiological observations and self-reported symptoms. For the purposes of this evaluation, the algorithms were designed to provide automated advice.
A trial run of the algorithms was performed by clinical staff; feedback from this process allowed us to modify and streamline the algorithms. A workshop day was organised through the NHS patient and public involvement, and volunteers provided feedback on the equipment, information packs and text-messaging. Despite some of the volunteers having never sent a text message before, they could communicate proficiently with FLO after a brief tutorial.
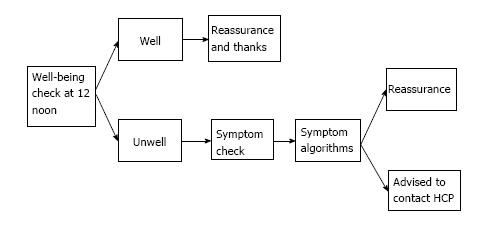
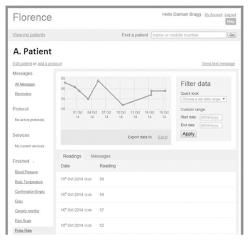
The well-being checks followed an algorithm, outlined in Figure 2. If patients reported feeling unwell, FLO would proceed to ask the patients for more specific information, limited to the symptoms set out in Table 1. An alert would also be highlighted on the clinicians’ FLO dashboard (Figure 3) for patients reporting feeling unwell, any complications, or abnormal physiological readings. Patients who developed an alert on the FLO dashboard were telephoned by the ERAS nurse practitioner within office hours. FLO has the capability to email or text the health care professional when an alert has been triggered. For this evaluation, alert forwarding to the ERAS team was not utilised.
| Symptoms | ||
| Nausea and vomiting | Stoma - constipation | Fever |
| Urinary | Emptying stoma bag | Generally unwell |
| Wound appearance | Bowels - loose stools | Tired |
| Painful wound | Bowels - constipated | Swollen leg |
| Stoma - loose motion | Pain | Shortness of breath/chest pain |
Algorithms were programmed to respond to symptoms that patients could text in to obtain advice. Each symptom required a separate algorithm to be constructed. The list of symptoms was provided as part of an information booklet that patients received, including a brief explanation of each symptom.
Upon receipt of a symptom, FLO proceeds to ask the patient clinical questions to identify a diagnosis. If the symptoms aligned with expected self-limiting problems, reassurance was provided, otherwise, advice was sent and an alert would be created on the clinician’s FLO dashboard.
As an example, for symptoms related to bowel movements, the patients would be asked to provide Bristol Stool Scale ratings. These are plotted on the FLO online dashboard, and management options are automatically sent to patients, for example, reduce opioid intake, contact the stoma nurses or increase fluid intake.
During the first 10 d after discharge, several reminder messages were sent to patients, including the timing of removal of surgical skin clips, the importance of regular mobilisation, dietary advice, and the nature of erratic bowel movement after colonic resections. It is also possible to remind patients to take medications at specific times, for example, extended venous thromboembolism prophylaxis.
Lead clinicians in local primary care and in the emergency department were consulted as to the nature of the service being evaluated. It was agreed that certain “trigger” conditions should inform the patient to present either to the colorectal department, the emergency department or to the patient’s general practitioner (Table 2).
| Colorectal team | Emergency department | General practitioner |
| Small bowel obstruction, postoperative ileus, retention of urine, hernia, surgical site infection, stoma problems, high output stoma, SIRS, fever, DVT, hypotension | Breathlessness, chest pain | Analgesia review, urinary tract infection |
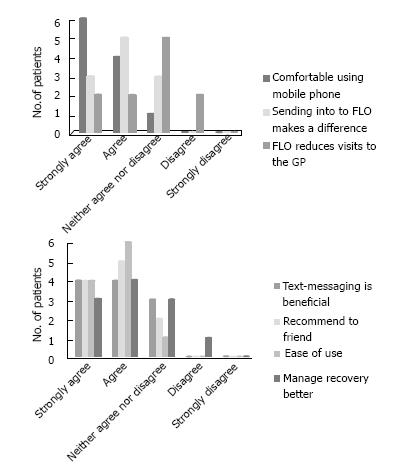
Patients were given packs consisting of: (1) Consent form; (2) generic FLO information leaflet; (3) colorectal ERAS FLO information booklet; (4) blood pressure cuff (providing blood pressure and pulse rate); (5) thermometer; and (6) evaluation forms (Likert 5-point scale[10]) included the statements: I feel comfortable using a mobile phone with FLO; I feel confident that sending my symptoms and readings to FLO makes a difference; Regular contact with FLO means I need to visit my GP less often; Using computers and text-messages to follow-up patients following discharge from hospital is beneficial; I would recommend FLO to a friend or family member; FLO is easy to use; FLO is helping me manage my own recovery better.
Patients and/or patient’s carers involved in the evaluation were shown how to perform and upload temperature, heart rate and blood pressure measurements to FLO and were asked to provide at least one reading per day. If they forgot to provide a reading, a text-message reminder was automatically sent by FLO to ask for this. Certain symptoms, for example, “generally unwell”, would also trigger FLO to request basic physiological readings from the patient. If readings were outside a predetermined range (which can be customised), a request was sent by FLO to repeat the readings. Depending on the readings received, advice was provided, and an alert would be created on the FLO dashboard. All communications between FLO and the patient are stored. If a patient texted in a message that was not understood by FLO, these can be seen and reviewed. This helped us to refine communications. It was also possible to send customised messages to patients and read any responses through the clinician’s dashboard.
The FLO dashboard is a web-based interface utilising 256-bit encryption. The dashboard (Figure 3) displays a list of all active patients (i.e., those who are still within their 30-d participation), and also those who have been “discharged” from the service, or had opted out. The dashboard has several tabs which display patients’ readings including: Well-being checks; basic observations; alerts generated; all support messages and any symptoms or free-text that the patient has sent.
During the 4-wk trial period, 24 patients were approached. Twenty patients were eligible to use the service. Two patients were eligible but declined to participate. Eighteen patients agreed to trial the service, but 1 did not opt in via text message and did not participate any further. Out of the 17 who opted in, 16 reliably interacted with the service at home. The patient who did not use the service after opting in was readmitted within 24 h of discharge. At any time after 4 d, patients could opt out of the service with no further ERAS follow up.
We subdivided the data into patients readmitted vs those not readmitted in Table 3. The mean follow up period is based on the number of days patients remained under FLO follow up before opting out. Patients were asked by FLO at noon daily whether they felt well or unwell. Whilst patients were opted in, the overall response rate to the well-being check was 83%, and the number of days patients were unwell in each category is demonstrated in Table 3. Abnormal observations of blood pressure, heart rate and temperature were defined according to the National Early Warning Score (NEWS)[11]. All symptoms reported by patients were recorded, including those not recognised by FLO (e.g., “anxiety shakes”). The number of symptoms reported are somewhat skewed in the readmitted group as one patient uploaded 50 symptoms (mainly due to pre-existing health conditions).
| No. of Patients | Total follow-up period (d) | Mean follow-up (d) | No. of days unwell (%) | Abnormal observations, n (%) | Symptoms reported, n (n/d) | |
| Not readmitted | 14 | 390 | 27.9 | 9 (3) | 21 (5) | 37 (0.09) |
| 3 | 34 | 11.3 | 14 (54) | 12 (18) | 55 (1.62) |
The numbers observed in this study are too small to draw any firm conclusions of any impact this technology could have. We did note that in the patients who were readmitted, more had uploaded abnormal observations (18% vs 5%), and reported being unwell on more of the days they were followed up for (54% vs 3%).
Most patients felt that the text-messaging service was acceptable to them and patient feedback about the service is summarised in Figure 4.
This investigation has demonstrated that it is feasible to develop an acceptable method of remotely monitoring patients who have undergone colorectal surgery after discharge. Using a basic telehealth solution, we designed advanced algorithms to monitor the daily well-being of patients, their physiological observations, and provided a method to respond and triage common postoperative symptoms and diagnoses. We also provided support messages for common postoperative problems. The method was feasible and acceptable to patients and reduced the number of telephone follow-up consultations required as part of our usual care.
Following discharge from hospital, patients’ care is effectively “handed back” to primary care services. However, despite the limited number of complications which can be dealt with in primary care (Table 2), there is a lack of incentive for secondary care teams to provide assistance for patients after discharge from hospital[12]. Patients discharged from hospital could thus be considered to be in “no-man’s land”. Advances in perioperative practice include strategies to reduce the magnitude and impact of surgical trauma, for example, by reducing inappropriate sympathetic response by thoracic epidural analgesia usage[13], and pre-loading patients with carbohydrate drinks to reduce postoperative insulin resistance[14]. Reducing the physiological burden that surgery places on patients permits a quicker recovery[15], reduced length of stay[16] and cost savings[17]. Although readmission to hospital may be viewed as a quality marker, the notion that patients are discharged prior to full recovery[1] more likely reflects advancements in treatment, as seen in ERAS programmes, where patients can be fit for discharge in as little as 23-48 h following major abdominal surgery[15,18].
Although no differences in readmission rates have been reported utilising ERAS pathways[19], the more serious complications, such as anastomotic leak, are reported to be diagnosed, on average, 12.7 d after surgery[20], and mortality resulting from this complication approaches 22%[21]. It is, therefore, important that signs of complications are recognised early, especially after discharge, to prevent patients from being readmitted in extremis.
When serious complications, such as anastomotic leaks, occur when patients are at home, treatment can be delayed. The use of the technology described in this trial may assist in identifying and treating complications sooner. At NUH, we have recently introduced “surgical hot clinics”, where appointments can be made by clinicians to review patients on a “very urgent” outpatient basis. Although the numbers in this feasibility trial were small, we have demonstrated that remote monitoring of patients after major abdominal surgery is possible, in what could be viewed as a “virtual ward”. Utilising a telehealth service may permit a more integrated and supportive discharge from secondary care, and could help to bridge the gap to a full primary care hand-back.
Prolonging hospital stay for patients who have apparently recovered would inevitably reduce readmissions, but this approach does not make financial sense, and exposes patients to additional risks associated with prolonged stay. Although epidemiologists have evaluated methods of predicting patients at higher risk for readmission[22], the scoring systems have not been widely adopted in United Kingdom surgical practice.
The algorithms in the current study were designed to be automated, but we felt the system was too complex given the limitations of the technology being utilised. For example, FLO can identify key words such as “bleeding” or “blood”, and an algorithm could be created to ask specific questions about where the bleeding is coming from, how much there is, and whether it’s mixed with anything else. The FLO “brain” has limited intelligence. It is not possible to program a “yes” or “no” response for individual symptom algorithms, as FLO cannot discriminate “yes” or “no” from other symptom algorithms. If bleeding was the symptom, FLO would have to ask: (1) Where the bleeding is coming from. Responses would have to be programmed and built in to the response sent to the patient (i.e., per rectal bleeding, per stomal bleeding, wound bleeding); (2) responses then must be carefully phrased in lay language, but are limited to 144 characters; and (3) for FLO to understand the reply, only specific phrases are understood, such as “B1” for rectal bleeding, “B2” for Stoma bleeding, or “B3” for Wound bleeding, which can be confusing, particularly if patients use phones where the previous messages are not on the same screen when they type their response.
FLO in its current guise requires patients to be precise in their responses; there is no “fuzzy matching”. For example, a patient may text in “lose bowls”, meaning loose bowels. FLO would respond to this with a generic “I didn’t understand that” or “that reading is too low”. It is possible to add additional keywords to individual algorithms to pick up potential misspellings or abbreviations, but this was not done during this short trial.
Finally, using FLO in an automated manner is not feasible. We felt that to comprehensively monitor patients remotely in this manner, a clinician is required to oversee the dashboard to ensure patients were not running into problems. Conversely, we did not have to make routine telephone calls to 13 of the 17 patients who utilised the service, which usually take approximately 20 min to complete. It is possible to provide shared cross-speciality access to the FLO dashboard, but since this was a small, short-term trial, we did not provide FLO access to individual GPs or the emergency department.
FLO could be used in other surgical practice including patients being sent home with drains - for example those with biliary or pancreatic fistulas, or after breast surgery in those with seromas.
The use of a modern technology was evaluated to remotely monitor patients who have undergone major abdominal surgery after discharge from hospital. The technology as it currently exists has limitations, and is not suitable for every patient. However, its use appears to be acceptable to those who did use it, and requires further evaluation as a method to bridge the gap between primary and secondary care services.
Patients can be fit for discharge in as little as 23-48 h following major abdominal surgery. However, serious complications, such as anastomotic leak, are reported to be diagnosed, on average, 12.7 d after surgery. It is therefore important that signs of complications are recognised early, to prevent patients from being readmitted in extremis. The aim of this telehealth evaluation was to monitor patients in the early follow up period after discharge.
Traditional follow-up after surgery included a telephone call from a nurse between 2-4 d following discharge to patients at home. This method is fairly resource-intense and does not include basic physiological parameters.
Although this evaluation was not powered to detect changes in the ability to pick up postoperative changes earlier, we have demonstrated that this method of follow-up is acceptable to patients and may form the basis of a larger study, which may incorporate newer technologies, such as the internet of things (IoT).
The evaluation of this technology demonstrates that use of telehealth in the immediate postoperative period is feasible and may help identify postoperative complications sooner. Methods arising from this evaluation may assist in future medical applications, such as devices in the IoT.
Telehealth is the provision of healthcare remotely by means of telecommunications technology.
This is a very well written article reporting an innovative approach for following patients after discharge.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fiori E, Majbar AM, Mayol J S- Editor: Ji FF L- Editor: A E- Editor: Zhao LM
| 1. | Kmietowicz Z. Hospitals will be fined for emergency readmissions, says Lansley. BMJ. 2010;340:c3079. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | NHS England PHE, Monitor, Care Quality Commission, Health Education England. Five year forward view. J Perioperat Pract. 2014;24:267. |
| 3. | Stewart J, Lloyd GM, Smith JK, Acheson AG, Williams JP, Maxwell-Armstrong CA. Could telephone reviews reduce readmission rates after laparoscopic colorectal surgery? Bull R Coll Surg Engl. 2012;94:162-164. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Cottrell E, Chambers R, O’Connell P. Using simple telehealth in primary care to reduce blood pressure: a service evaluation. BMJ Open. 2012;2:pii: e001391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Head BA, Keeney C, Studts JL, Khayat M, Bumpous J, Pfeifer M. Feasibility and Acceptance of a Telehealth Intervention to Promote Symptom Management during Treatment for Head and Neck Cancer. J Support Oncol. 2011;9:e1-e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Semple JL, Sharpe S, Murnaghan ML, Theodoropoulos J, Metcalfe KA. Using a mobile app for monitoring post-operative quality of recovery of patients at home: a feasibility study. JMIR Mhealth Uhealth. 2015;3:e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Chi NC, Demiris G. A systematic review of telehealth tools and interventions to support family caregivers. J Telemed Telecare. 2015;21:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 8. | Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH; CONNECT Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 375] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 9. | Hammond JS, Humphries S, Simson N, Scrimshaw H, Catton J, Gornall C, Maxwell-Armstrong C. Adherence to enhanced recovery after surgery protocols across a high-volume gastrointestinal surgical service. Dig Surg. 2014;31:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22:5-55. |
| 11. | Goldhill DR, McNarry AF. Physiological abnormalities in early warning scores are related to mortality in adult inpatients. Br J Anaesth. 2004;92:882-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Roland M, Abel G. Reducing emergency admissions: are we on the right track? BMJ. 2012;345:e6017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Holte K, Kehlet H. Epidural anaesthesia and analgesia - effects on surgical stress responses and implications for postoperative nutrition. Clin Nutr. 2002;21:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Nygren J, Thorell A, Ljungqvist O. Preoperative oral carbohydrate nutrition: an update. Curr Opin Clin Nutr Metab Care. 2001;4:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Rossi G, Vaccarezza H, Vaccaro CA, Mentz RE, Im V, Alvarez A, Quintana GO. Two-day hospital stay after laparoscopic colorectal surgery under an enhanced recovery after surgery (ERAS) pathway. World J Surg. 2013;37:2483-2489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 825] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 17. | Stowers MD, Lemanu DP, Hill AG. Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth. 2015;62:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Levy BF, Scott MJ, Fawcett WJ, Rockall TA. 23-hour-stay laparoscopic colectomy. Dis Colon Rectum. 2009;52:1239-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 628] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 20. | Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 447] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 641] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 22. | Billings J, Blunt I, Steventon A, Georghiou T, Lewis G, Bardsley M. Development of a predictive model to identify inpatients at risk of re-admission within 30 days of discharge (PARR-30). BMJ Open. 2012;2:pii: e001667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |