Published online Sep 27, 2017. doi: 10.4240/wjgs.v9.i9.186
Peer-review started: January 18, 2017
First decision: March 6, 2017
Revised: March 24, 2017
Accepted: July 7, 2017
Article in press: July 10, 2017
Published online: September 27, 2017
Processing time: 252 Days and 12.2 Hours
The evolution of multi-visceral and isolated intestinal transplant techniques over the last 3 decades has highlighted the technical challenges related to the closure of the abdomen at the end of the procedure. Two key factors that contribute to this challenge include: (1) Volume/edema of donor graft; and (2) loss of abdominal domain in the recipient. Not being able to close the abdominal wall leads to a variety of complications and morbidity that range from complex ventral hernias to bowel perforation. At the end of the 90’s this challenge was overcome by graft reduction during the donor operation or bench table procedure (especially reducing liver and small intestine), as well as techniques to increase the volume of abdominal cavity by pre-operative expansion devices. Recent reports from a few groups have demonstrated the ability of transplanting a full-thickness, vascularized abdominal wall from the same donor. Thus, a spectrum of techniques have co-evolved with multi-visceral and intestinal transplantation, ranging from graft reduction to enlarging the volume of the abdominal cavity. None of these techniques are free from complications, however in large-volume centers the combinations of both (graft reduction and abdominal widening, sometimes used in the same patient) could decrease the adverse events related to recipient’s closure, allowing a faster recovery. The quest for a solution to this unique challenge has led to the proposal and implementation of innovative solutions to enlarge the abdominal cavity.
Core tip: Matching donors with recipients to perform liver-bowel transplantation is a challenging task, especially in front of pediatric candidates due to the shortage of suitable donors. Historically, the issue was overcome reducing the size of liver and bowel during donation in order to implant the combined graft in the small abdominal cavity of the recipient. Due to the presence of complications, the procedure has been improved by enlarging the abdominal cavity of the recipients, initially through conventional techniques used in hernia repair or trauma surgery and later by transplanting the donor abdominal wall into the recipient. Results are encouraging but limited to high experienced centers.
- Citation: Lauro A, Vaidya A. Role of “reduced-size” liver/bowel grafts in the “abdominal wall transplantation” era. World J Gastrointest Surg 2017; 9(9): 186-192
- URL: https://www.wjgnet.com/1948-9366/full/v9/i9/186.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i9.186
Experience has shown that intestinal and multi-visceral transplantation (ITx) is a feasible and potentially life-saving procedure. Donor-recipient size discrepancies have however been the Achilles heel, limiting the pool of donor organs especially for pediatric recipients due to donor-to-recipient body weight ratio, ideally between 1.1 and 0.76[1] , and size mismatching makes primary closure of the recipient abdominal wall one of the important technical challenges related to intestinal transplantation, mainly due to two factors: loss of abdominal domain because of sepsis, enteric-cutaneous fistulas or multiple surgeries of the recipient[2]; and volume or post-reperfusion intestinal edema of the graft[3]. Achieving tension-free closure after bowel transplant is of utmost importance to avoid post-operative abdominal compartment syndrome, risking ischemia and necrosis of the graft[4].
Different options have been reported in literature when a fascia closure is impossible, in the case of a donor-recipient size mismatch that has been undertaken due to unavailability of smaller donors.
The two main approaches focus on (1) Volume reduction of the graft[5]; or (2) an enlargement of the recipient abdominal domain[6]. The first approach includes an anatomical reduction of the graft that mainly applies for pediatric transplantation to prevent high waitlist mortality rates, while the second approach focuses on techniques to enlarge the abdominal domain ,mainly used in > 18 years population.
Pre-transplant mortality has gradually decreased for pediatric candidates in United States (less than 3 per 100 waitlist years, while for adult candidates is at 22.1 per 100 waitlist years), but notably it is still higher for intestine-liver transplant candidates[7], especially represented by the pediatric population: The need of total parenteral nutrition puts children at risk for developing liver disease and subsequently life-threatening complications[8].
Since the 90’s, the conventional transplant approach has utilized small size donors. But given the shortage of donors that fulfill the ideal characteristics, transplant centers have been increasingly accepting organs with considerable graft mismatch. Reducing the size of transplanted organs, with a reduced-size composite liver-intestinal allograft using split techniques[9], has resulted in utilization of organs from donors up to five times larger than recipients[10].
The development of reduced-size isolated bowel grafts has improved the limited availability of donors for candidates weighing less than 10 kg due to the possibility to overcome donor-recipient size mismatches[11] greater than to 10:1 (body weight).
An alternate method to solve the issue of size mismatch involves abdominal wall reconstruction, enabling substantial expansion of the recipient’s abdominal domain, especially when more organs (like liver-bowel) are to be transplanted[12]. However, this is challenging since most recipients are poor candidates for plastic surgery techniques such as tissue advancement or flap closure of the defect because of many previous surgeries.
Few techniques of abdominal wall reconstruction have been reported, many of them already used in difficult abdominal wall hernia repair or trauma surgery. Staged closure of the abdomen has been described by the Birmingham (United Kingdom) group[13], reporting on 23 combined liver and bowel transplants closed using a Silastic® sheet together with a vacuum occlusive dressing.
The skin of the abdominal wall is often more pliable than the underlying tissue, and closure is possible sometimes with the help of tissue expanders[14,15]: Accordingly, twenty cases of inflatable tissue expanders in ITx candidates were reported in international literature. Localization of tissue expanders were: Subcutaneously in 13; intraperitoneally in 4; placed retromuscularly and 1 intraperitoneally; 1 patient had biplanar tissue expander (intraperitoneally placed and extending retromuscularly) and in 1 localization was unreported.
Alternatively, common used techniques include absorbable mesh[16]: Five pediatric liver and intestinal living-donor transplant recipients were treated by Chicago group initially through an absorbable Polygalactin mesh and later , once a granulated tissue was present, by a split-thickness skin graft. Sometimes the use of non-absorbable mesh[17] has also been reported: a prosthetic mesh alone was used in three patients from Bologna series to perform abdominal reconstruction , only in one case followed by a myocutaneous flap.
Apart from traditional reconstructive techniques, alternative methods include bioengineered skin equivalent[18], a-cellular dermal matrix[19,20], frozen human fibroblast-derived dermis[21], non-vascularized rectus muscle fascia[22,23], and vascularized “split-thickness”[24] or “full-thickness” skin grafts[25-31], either with classical[25], microsurgical[32] or remote revascularization technique[33]. These techniques are summarized in Table 1.
| Ref. | Children/adults with difficult closure | Techniques used for closure | Post-ITx complications related to closure |
| Nery et al[5], 1998 | N.a./n.a. tot = 11 (+ 5 graft reduction/modification) | 4 silastic or PTFE mesh | 5 incomplete closure |
| 2 skin flap | |||
| 1 myocutaneous flap | |||
| 3 mesh + graft reduction | |||
| 1 skin flap + graft reduction | |||
| Alexandrides et al[4], 2000 | 9/6 | 7 goretex mesh | None |
| 4 myocutaneous flap | |||
| 3 silastic mesh | |||
| 1 abdominal expander | |||
| Levi et al[25], 2003 | 2/6 | 8 full-thickness wall graft | 2 wall infarction |
| Charles et al[21], 2004 | 0/1 | 1 fibroblast-derived dermis | None |
| Drosou et al[18], 2005 | 0/4 | 4 bioengineered skin equivalent | None |
| Asham et al[19], 2006 | 0/1 | 1 acellular dermal matrix | None |
| Carlsen et al[2], 2007 | 8/6 | 7 goretex mesh | 6 incisional hernia |
| 4 (+ 2) split-thickness skin graft | |||
| 2 (+ 2) skin flap | |||
| 1 (+ 1) fascia | |||
| Zanfi et al[3], 2008 | 0/13 (+ 2 graft reduction) | 5 skin closure | 6 incisional hernia |
| 1 staged closure | 4 mesh infection | ||
| 4 prosthetic mesh | 2 fistulas | ||
| 3 full-thickness wall graft | 1 abdominal compartments | ||
| Gondolesi et al[22], 2009 | 10/6 | 16 non-vascularized rectus fascia | 7 wall infections |
| Grevious et al[16], 2009 | 5/0 | 5 staged closure (meshà split-thickness skin graft) | 1 fistula |
| Sheth et al[13], 2012 | 23/0 | 23 staged closure | 2 abdominal compartment s. |
| Mangus et al[20], 2012 | 12/25 | 30 acellular dermal allograft | 1 dehiscence |
| 7 mesh or donor fascia | 5 incisional hernia | ||
| 2 fistulas | |||
| Vianna et al, 2013 (unpublished results) | 0/1 | 1 full-thickness wall graft | N.a. |
| Weiner et al[15],2014 | 1/0 | 1 bi-planar tissue expander | None |
| Vaidya et al, 2015 (in Chennai) (unpublished results) | 1 n.a. | 1 full-thickness wall graft | N.a. |
| Haveman et al[35], 2016 | 0/1 | 1 full-thickness wall graft | None |
| Giele et al[24], 2016 | 0/19 | 17 full-thickness wall graft | 3 wound infection |
| 1 partial-thickness vascularized graft 1 partial-Thickness nonvascularized graft |
The use of either vascularized “partial” (rectus fascia) or “full-thickness” abdominal wall insensate[34] grafts (obtained from the same donor as the intestinal organs) has been successfully done in both, adult[35] as well as pediatric population[25].
The vascularized donor abdominal wall may have an immunological impact as well[36], and it has been proposed as a “sentinel” graft[37-41]. An allograft skin rash may represent a rejection phenomenon occurring earlier than the bowel manifestations, allowing to minimize therapy because treatment of abdominal wall rejection (very often steroid-responsive) may prevent intestinal rejection, which is a much more difficult issue to handle pharmacologically.
It has been hypothesized that the combined skin-intestine allograft from the same donor could present diagnostic and therapeutic advantages to the patient and clinician. Furthermore it has also reported the benefit of the skin, from the vascularized abdominal wall, being used to detect graft versus host disease in recipients of a combined abdominal wall-bowel graft by identifying a body rash in the recipient that spares the skin of the abdominal wall graft[42,43].
Procurement strategies for combined multi-organ and composite tissues for transplantation[44] continue to evolve, from the initial reports back in the early 90’s. In case of donor-recipient size mismatch[5], the surgeon could reduce the graft or conversely retrieve an abdominal wall during donor operation.
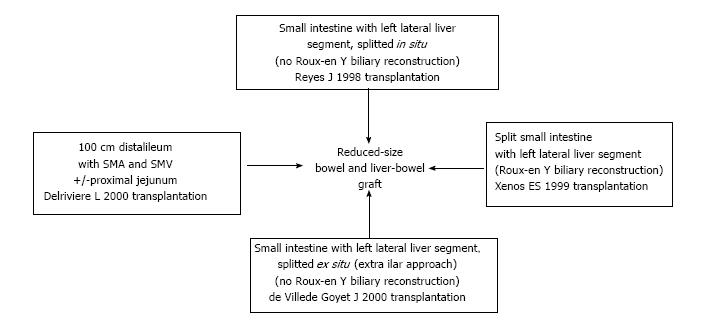
Splitting both liver (left lateral segment represented by segments II and III) and intestine (ileum) during a combined transplantation, with resulting Roux-en-Y loop biliary reconstruction in the recipient, was first reported by Xenos et al[45] in 1999.
Another way to reduce the liver-bowel graft during the harvest was described by Reyes et al[9] isolating the intestine and removing it en-block with the left lateral liver segment (segment II and III, previously splitted in situ): Eliminating the need of biliary reconstruction reduces most technical complications and avoids the use of the bowel for bilio-digestive anastomosis.
A similar advantage was reported by de Ville de Goyet et al[10], where during the bench table surgery the liver was reduced, using an approach that leaves the liver hilum untouched.
Isolated intestinal grafts could be size-modified: Fifteen small bowels were successfully reduced by Delrivière et al[11] obtaining a one meter ileal graft vascularized by the superior mesenteric artery and vein. Later, technical modifications allowed the use of two grafts from a single donor, represented by part of ileum and part of jejunum.
These techniques are summarized in Figure 1.
Two popular procedures have been reported in order to harvest an abdominal wall: In the original Miami technique[25] the vessels of the wall graft were represented by donor femoral and iliac vessels, together with a small patch of aorta and inferior vena cava used to implant them into the recipient’s common iliac artery and vein. A modified microsurgical procedure was later reported by Cipriani et al from Bologna[32], collecting only the donor epigastric vessels with the abdominal wall, so sparing donor femoral-iliac vascular axes by direct anastomosis of the inferior donor-recipient epigastric vessels.
Both the procedures (size reduction and abdominal wall retrieval) are time-consuming in both donor and recipient operations but it is worthwhile to notice that, to date, there have been only insensate abdominal wall graft retrievals without nerve coaptation, a factor that may further impact procurement time if added in the future[46].
The “golden age” of the reduced size techniques was practiced till the back end of the 90’s. In 1998 Reyes et al[9] reported the cases of a 3-year-old boy with hepatic-intestinal failure and a 63-year-old man with a central hepatoma and hepatitis C cirrhosis, both transplanted using the same adult cadaveric donor. The donor left lateral hepatic segment (segment II and III) in continuity with the small intestine was implanted into the child, using a modified in situ split technique where biliary reconstruction is unnecessary, while the right side of the donor liver was transplanted into the man. The pediatric recipient was later re-transplanted due to a liver damage related to a native pancreatic fistula, while the adult patient died for rupture of pseudo-aneurysm related to infection of the arterial graft.
In 1999 Xenos et al[45] described the use in a child of split liver (left lateral segment represented by segment II and III) and partial intestine (ileum) from a cadaveric donor during a combined transplantation: The right side went to an adult discharged home without complications. The pediatric recipient underwent a Roux-en-Y loop biliary reconstruction: Later he died for intestinal perforation plus severe rejection.
In 2000, de Ville de Goyet et al[10] transplanted two children, weighing 7.6 and 9.8 kg respectively, with a composite graft procured from donors weighing 35 kg (almost five times larger): Both went home on full enteral feeds. The composite graft was obtained during bench table surgery (leaving the hepatic hilum untouched) and was represented by liver segment II and III and whole small bowel, including duodenum and pancreas head. Also in this case there was no need of biliary reconstruction due to the preservation of the donor duodenum in continuity with the combined graft.
At the beginning of the new millennium, a rather innovative method to overcome the donor-recipient size-mismatching was hypothesized and VCA (vascularized composite allograft) was first reported by Levi et al[25] in 2003 in the form of abdominal wall transplantation: Their idea was to cover at the end of an ITx the resulting abdominal wall defect with both donor rectus abdominis muscles plus fascia, subcutaneous tissue and skin. The Miami group transplanted the wall graft like a kidney allograft, using as a blood supply the donor inferior epigastric vessels (left in continuity with the femoral and iliac vessels), and implanting them into the recipient’s common iliac artery and vein. The procedure time was about 2 h and this full thickness, vascularized, myocutaneous free flap was finally rotated and positioned according to location of the abdominal wall defect. Doppler ultrasound was used to monitor the blood flow.
The procedure was later modified by the Bologna group[32], using a microsurgical technique with a Zeiss microscope (Oberkochen; Germany): The donor epigastric pedicles were anastomosed end-to-end with the recipient epigastric vessels with no need to collect the donor femoral and iliac vessels. The operative time was similar to the one reported by Miami group.
Giele et al[33] from Oxford (United Kingdom) faced a different issue related to abdominal wall transplantation: The storage and subsequent ischemia-reperfusion injury of the wall graft during > 5 h ITx procedures. The ischemic time was minimized by two teams working at the same time on the recipient, one performing the intestinal transplant and the other re-vascularizing the abdominal wall remotely on the recipient forearm vessels. The procedure time lasted 50 min (30-60 min). Later the wall graft was re-vascularized on the abdomen.
Other groups reported, even very recently, few cases of abdominal wall transplantation[35] but the comprehensive picture of the results, related to the use of VCAs to close the abdominal wall after intestinal/multi-visceral transplantation ,were summarized in a recent paper published in 2017[24] where 35 full-thickness vascularized abdominal wall transplants were described (17 in Oxford, 12 in Miami, 3 in Bologna, 1 in Chennai, 1 in Indianapolis, 1 in Groningen).
The reported rate of successful abdominal closure after abdominal wall transplantation is very high, with 88% of flap/graft survival and no related mortality[26]: The overall follow-up is between 6 mo (Oxford, Bologna) and 7 years (Miami).
Moreover, it is worthwhile to notice that the skin component of the abdominal wall may serve as an immune modulator: A recent paper[37] analyzed a small cohort of 29 intestinal/multi-visceral transplants, 14 of them combined with abdominal wall transplants. The advantage to carry a wall graft was represented by lower bowel rejection rate (7% vs 27%) and lower rate (14% vs 33%) of misdiagnoses (viral infection vs rejection), followed by better intestinal graft survival (79% vs 60%).
Despite the good outcome, the procedure is still limited in few transplant centers where the expertise of the transplant team is well integrated with the plastic surgical service: Due to the low the numbers presented also by the 3 main groups (Miami, Oxford and Bologna) it is not possible to make a definitive statement related to the best technique (less morbidity, flap loss, and operative time).
Literature has shown that wall transplantation is feasible and reasonably time-consuming but it is a safe procedure with low morbidity and mortality.
The evolution and success of intestinal and multi-visceral transplantation has, in the last 20 years, raised the issue of difficult or even impossible abdominal closure, a topic very rarely encountered in other fields of transplantation.
The number of transplanted organs (volume) and/or graft edema, worsened by a small recipient abdominal cavity due to age or previous surgeries, makes a primary closure technically challenging or even impossible.
Different techniques have been proposed to address this topic and the choice depends upon the transplant team’s expertise and/or the availability of a plastic surgical service.
Whatever the approach used, may it be reduction of donor graft size or abdominal wall transplantation, it is important to realize that they may not be mutually exclusive to each other and both approaches can be used as a combination in the same recipient to assure the success of the transplant procedure.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fogli L S-Editor: Gong ZM L-Editor: A E-Editor: Zhao LM
| 1. | Fishbein TM, Bodian CA, Miller CM. National sharing of cadaveric isolated intestinal allografts for human transplantation: a feasibility study. Transplantation. 2000;69:859-863. [PubMed] |
| 2. | Carlsen BT, Farmer DG, Busuttil RW, Miller TA, Rudkin GH. Incidence and management of abdominal wall defects after intestinal and multivisceral transplantation. Plast Reconstr Surg. 2007;119:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Zanfi C, Cescon M, Lauro A, Dazzi A, Ercolani G, Grazi GL, Del Gaudio M, Ravaioli M, Cucchetti A, La Barba G. Incidence and management of abdominal closure-related complications in adult intestinal transplantation. Transplantation. 2008;85:1607-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Alexandrides IJ, Liu P, Marshall DM, Nery JR, Tzakis AG, Thaller SR. Abdominal wall closure after intestinal transplantation. Plast Reconstr Surg. 2000;106:805-812. [PubMed] |
| 5. | Nery JR, Weppler D, DeFaria W, Liu P, Romero R, Tzakis AG. Is the graft too big or too small? Technical variations to overcome size incongruity in visceral organ transplantation. Transplant Proc. 1998;30:2640-2641. [PubMed] |
| 6. | Gerlach UA, Pascher A. Technical advances for abdominal wall closure after intestinal and multivisceral transplantation. Curr Opin Organ Transplant. 2012;17:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Smith JM, Skeans MA, Horslen SP, Edwards EB, Harper AM, Snyder JJ, Israni AK, Kasiske BL. Intestine. Am J Transplant. 2017;16 Suppl 2:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ganousse-Mazeron S, Lacaille F, Colomb-Jung V, Talbotec C, Ruemmele F, Sauvat F, Chardot C, Canioni D, Jan D, Revillon Y. Assessment and outcome of children with intestinal failure referred for intestinal transplantation. Clin Nutr. 2015;34:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Reyes J, Fishbein T, Bueno J, Mazariegos G, Abu-Elmagd K. Reduced-size orthotopic composite liver-intestinal allograft. Transplantation. 1998;66:489-492. [PubMed] |
| 10. | de Ville de Goyet J, Mitchell A, Mayer AD, Beath SV, McKiernan PJ, Kelly DA, Mirza D, Buckles JA. En block combined reduced-liver and small bowel transplants: from large donors to small children. Transplantation. 2000;69:555-559. [PubMed] |
| 11. | Delrivière L, Muiesan P, Marshall M, Davenport M, Dhawan A, Kane P, Karani J, Rela M, Heaton N. Size reduction of small bowels from adult cadaveric donors to alleviate the scarcity of pediatric size-matched organs: an anatomical and feasibility study. Transplantation. 2000;69:1392-1396. [PubMed] |
| 12. | Panaro F, Ornis S. Abdominal wound closure in liver-intestine pediatric transplantation. Pediatr Transplant. 2009;13:654-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Sheth J, Sharif K, Lloyd C, Gupte G, Kelly D, de Ville de Goyet J, Millar AJ, Mirza DF, Chardot C. Staged abdominal closure after small bowel or multivisceral transplantation. Pediatr Transplant. 2012;16:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Ceulemans LJ, Deferm NP, Miserez M, Maione F, Monbaliu D, Pirenne J. The role of osmotic self-inflatable tissue expanders in intestinal transplant candidates. Transplant Rev (Orlando). 2017;30:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Weiner J, Wu J, Martinez M, Lobritto S, Ovchinsky N, Rohde C, Griesemer A, Kato T. The use of bi-planar tissue expanders to augment abdominal domain in a pediatric intestinal transplant recipient. Pediatr Transplant. 2014;18:E174-E179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Grevious MA, Iqbal R, Raofi V, Beatty E, Oberholzer J, Cohen M, Abcarian H, Testa G, Benedetti E. Staged approach for abdominal wound closure following combined liver and intestinal transplantation from living donors in pediatric patients. Pediatr Transplant. 2009;13:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Di Benedetto F, Lauro A, Masetti M, Cautero N, De Ruvo N, Quintini C, Diago Uso’ T, Romano A, Dazzi A, Ramacciato G. Use of prosthetic mesh in difficult abdominal wall closure after small bowel transplantation in adults. Transplant Proc. 2005;37:2272-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Drosou A, Kirsner RS, Kato T, Mittal N, Al-Niami A, Miller B, Tzakis AG. Use of a bioengineered skin equivalent for the management of difficult skin defects after pediatric multivisceral transplantation. J Am Acad Dermatol. 2005;52:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Asham E, Uknis ME, Rastellini C, Elias G, Cicalese L. Acellular dermal matrix provides a good option for abdominal wall closure following small bowel transplantation: a case report. Transplant Proc. 2006;38:1770-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Mangus RS, Kubal CA, Tector AJ, Fridell JA, Klingler K, Vianna RM. Closure of the abdominal wall with acellular dermal allograft in intestinal transplantation. Am J Transplant. 2012;12 Suppl 4:S55-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Charles CA, Kato T, Tzakis AG, Miller BN, Kirsner RS. Use of a living dermal equivalent for a refractory abdominal defect after pediatric multivisceral transplantation. Dermatol Surg. 2004;30:1236-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Gondolesi G, Selvaggi G, Tzakis A, Rodríguez-Laiz G, González-Campaña A, Fauda M, Angelis M, Levi D, Nishida S, Iyer K. Use of the abdominal rectus fascia as a nonvascularized allograft for abdominal wall closure after liver, intestinal, and multivisceral transplantation. Transplantation. 2009;87:1884-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Gondolesi G, Fauda M. Technical refinements in small bowel transplantation. Curr Opin Organ Transplant. 2008;13:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Giele H, Vaidya A, Reddy S, Vrakas G, Friend P. Current state of abdominal wall transplantation. Curr Opin Organ Transplant. 2017;21:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Levi DM, Tzakis AG, Kato T, Madariaga J, Mittal NK, Nery J, Nishida S, Ruiz P. Transplantation of the abdominal wall. Lancet. 2003;361:2173-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Berli JU, Broyles JM, Lough D, Shridharani SM, Rochlin D, Cooney DS, Lee WP, Brandacher G, Sacks JM. Current concepts and systematic review of vascularized composite allotransplantation of the abdominal wall. Clin Transplant. 2013;27:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Diaz-Siso JR, Bueno EM, Sisk GC, Marty FM, Pomahac B, Tullius SG. Vascularized composite tissue allotransplantation--state of the art. Clin Transplant. 2013;27:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Knobloch K, Rennekampff HO, Meyer-Marcotty M, Gohritz A, Vogt PM. [Organ transplantation, composite tissue allotransplantation, and plastic surgery]. Chirurg. 2009;80:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Selvaggi G, Levi DM, Kato T, Madariaga J, Moon J, Nishida S, Tzakis AG. Expanded use of transplantation techniques: abdominal wall transplantation and intestinal autotransplantation. Transplant Proc. 2004;36:1561-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Selvaggi G, Levi DM, Cipriani R, Sgarzani R, Pinna AD, Tzakis AG. Abdominal wall transplantation: surgical and immunologic aspects. Transplant Proc. 2009;41:521-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Tzakis AG, Tryphonopoulos P, Kato T, Nishida S, Levi DM, Nery JR, Madariaga J, De Faria W, Mittal N, Thompson JF. Intestinal transplantation: advances in immunosuppression and surgical techniques. Transplant Proc. 2003;35:1925-1926. [PubMed] |
| 32. | Cipriani R, Contedini F, Santoli M, Gelati C, Sgarzani R, Cucchetti A, Lauro A, Pinna AD. Abdominal wall transplantation with microsurgical technique. Am J Transplant. 2007;7:1304-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Giele H, Bendon C, Reddy S, Ramcharan R, Sinha S, Friend P, Vaidya A. Remote revascularization of abdominal wall transplants using the forearm. Am J Transplant. 2014;14:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Mannu GS, Vaidya A. Thermal trauma to abdominal wall vascularised composite allotransplant. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Haveman JW, Tempelman TM, Hofker HS, Khoe PC, Dijkstra G, Werker PM. [First combined intestinal and abdominal wall transplantation in the Netherlands]. Ned Tijdschr Geneeskd. 2017;160:A9788. [PubMed] |
| 36. | Bejarano PA, Levi D, Nassiri M, Vincek V, Garcia M, Weppler D, Selvaggi G, Kato T, Tzakis A. The Pathology of full-thickness cadaver skin transplant for large abdominal defects: a proposed grading system for skin allograft acute rejection. Am J Surg Pathol. 2004;28:670-675. [PubMed] |
| 37. | Gerlach UA, Vrakas G, Sawitzki B, Macedo R, Reddy S, Friend PJ, Giele H, Vaidya A. Abdominal Wall Transplantation: Skin as a Sentinel Marker for Rejection. Am J Transplant. 2017;16:1892-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Allin BS, Ceresa CD, Issa F, Casey G, Espinoza O, Reddy S, Sinha S, Giele H, Friend P, Vaidya A. A single center experience of abdominal wall graft rejection after combined intestinal and abdominal wall transplantation. Am J Transplant. 2013;13:2211-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Barnes J, Issa F, Vrakas G, Friend P, Giele H. The abdominal wall transplant as a sentinel skin graft. Curr Opin Organ Transplant. 2017;21:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Ali JM, Catarino P, Dunning J, Giele H, Vrakas G, Parmar J. Could Sentinel Skin Transplants Have Some Utility in Solid Organ Transplantation? Transplant Proc. 2017;48:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Mannu GS, Vaidya A. An interesting rash following bowel and abdominal wall transplantation. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Lauro A, Arpinati M, Zanfi C, Morelli MC, D’Errico-Grigioni A, Bagni A, Dazzi A, Pironi L, Pinna AD. Extracorporeal photopheresis for chronic GVHD: case report after adult bowel-abdominal wall transplantation. Transplantation. 2013;96:e9-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Mannu GS, Vaidya A. Graft versus host disease following small bowel and abdominal wall transplantation. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Datta N, Yersiz H, Kaldas F, Azari K. Procurement strategies for combined multiorgan and composite tissues for transplantation. Curr Opin Organ Transplant. 2015;20:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Xenos ES, Khan F, Nery J, Romero R, Mocros J, Tzakis A. Cadaveric small bowel/split liver transplantation in a child. Transpl Int. 1999;12:63-67. [PubMed] |
| 46. | Broyles JM, Sarhane KA, Tuffaha SH, Cooney DS, Lee WP, Brandacher G, Sacks JM. Reconstruction of Large Abdominal Wall Defects Using Neurotized Vascular Composite Allografts. Plast Reconstr Surg. 2015;136:728-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |