Published online Jul 27, 2017. doi: 10.4240/wjgs.v9.i7.161
Peer-review started: December 30, 2016
First decision: January 28, 2017
Revised: May 26, 2017
Accepted: June 6, 2017
Article in press: June 8, 2017
Published online: July 27, 2017
Processing time: 208 Days and 22 Hours
To investigate predictors of perforation after endoscopic resection (ER) for duodenal neoplasms without a papillary portion.
This was a single-center, retrospective, cohort study conducted between April 2003 and September 2014. A total of 54 patients (59 lesions) underwent endoscopic mucosal resection (EMR) (n = 36) and endoscopic submucosal dissection (ESD) (n = 23). Clinical features, outcomes, and predictors of perforation were investigated.
Cases of perforation occurred in eight (13%) patients (95%CI: 4.7%-22.6%). Three ESD cases required surgical management because they could not be repaired by clipping. Delayed perforation occurred in two ESD cases, which required surgical management, although both patients underwent prophylactic clipping. All patients with perforation who required surgery had no postoperative complications and were discharged at an average of 13.2 d after ER. Perforation after ER showed a significant association with a tumor size greater than 20 mm (P = 0.014) and ESD (P = 0.047).
ESD for duodenal neoplasms exceeding 20 mm may be associated with perforation. ESD alone is not recommended for tumor treatment, and LECS should be considered as an alternative.
Core tip: Duodenal neoplasms are relatively rare, and endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) of the duodenum poses a high risk of complications. In our study, 54 patients (59 lesions) underwent EMR (n = 36) and ESD (n = 23). Cases of perforation occurred in eight (13%) patients (95%CI: 4.7%-22.6%), and perforation showed a significant association with a tumor size greater than 20 mm (P = 0.014) and ESD (P = 0.047). ESD for duodenal neoplasms exceeding 20 mm may be associated with perforation. ESD alone is not recommended as a treatment for tumor treatment, and laparoscopic and endoscopic cooperative surgery should be considered as an alternative.
- Citation: Matsuda Y, Sakamoto K, Kataoka N, Yamaguchi T, Tomita M, Makimoto S. Perforation associated with endoscopic submucosal dissection for duodenal neoplasm without a papillary portion. World J Gastrointest Surg 2017; 9(7): 161-166
- URL: https://www.wjgnet.com/1948-9366/full/v9/i7/161.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i7.161
Duodenal neoplasms are relatively rare. Duodenal polyps are found in 4.6% of patients referred for upper gastrointestinal endoscopy[1]. Primary adenocarcinoma represents only 0.3% of all gastrointestinal tract malignant neoplasms and 0.042% of all malignant neoplasms[2,3]. Therefore, no method of treatment for duodenal neoplasm has been established.
Recently, cases of endoscopic resection (ER) for superficial neoplasms without lymph node metastasis have been reported. ER may consist of endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). However, ER for the duodenum poses a high risk of complications, such as perforation and bleeding, due to the abundant blood vessels in the submucosal layer and thin muscle layer in the duodenum compared with the digestive tract[4-7]. Specifically, patients with perforation undergo emergency surgery in many cases, and it is unclear whether ER for duodenal tumors is appropriate. In this study, we investigated predictors of perforation after ER for duodenal neoplasms without a papillary portion.
This study included a retrospective cohort of 54 patients (59 lesions) in a single center. We recruited patients (without ampullary duodenal tumors) who underwent ER between April 2003 and September 2014. These patients were preoperatively diagnosed with adenoma or carcinoma. The database included patient information such as age, sex, treatment method (EMR or ESD), prophylactic clipping (applied or not applied), and tumor characteristics, such as histological diagnosis (adenoma or carcinoma), location (pre-ampulla or post-ampulla), size (under 20 mm or over 20 mm), and type (polyploid or superficial). When a patient had multiple duodenal tumors, the largest lesion was included in the analysis. When a tumor was located on the opposite side of the ampulla of Vater, it was categorized as post-ampullary. The clinical features of complications (perforation and bleeding) were investigated.
All patients were provided with an explanation of the endoscopic procedure before treatment, including complications and alternative treatments, and written informed consent was obtained.
The endoscopic procedures were performed with a single-channel endoscope (GIF-Q240 or PCF-PQ260I; Olympus Medical Systems Co., Tokyo, Japan) or a double balloon sigmoid scope (EN-450T5/W; FUJIFILM, Saitama, Japan) by carbon dioxide insufflation. The choice of scope depended on the distance to the lesion.
EMR was indicated for small lesions (< 2 cm) or pedunculated lesions. Simple snarectomy was performed after the injection of 0.4% sodium hyaluronate solution (MucoUp; Johnson and Johnson K.K., Tokyo, Japan). The mucosa bulge is important for the safety of the procedure because the wall of the duodenum is thin. ESD was indicated for large lesions (≥ 2 cm) or flattened lesions. The ESD technique consisted of three steps. First, the periphery of the lesion was marked using a 2.0 mm short needle knife with a water jet function (Flush Knife, DK2618JB20; FUJIFILM, Saitama, Japan). Second, MucoUp was injected into the submucosal layer to achieve sufficient mucosal elevation. Third, a mucosal incision and submucosal dissection were performed with the Flush Knife (1.5 mm or 2.0 mm). Additionally, an electric current generator (VIO300D; ERBE, Tübingen, Germany) was used for hemostasis.
Prophylactic clipping using hemoclips (HX-110/610; Olympus Medical Systems Co.) was performed for mucosal defects after ER.
Intraoperative perforation was defined as the ability to recognize a perforation during the EMR and ESD procedures. Delayed perforation was defined as the inability to recognize a perforation during the EMR and ESD procedures, and patients had no symptoms immediately after the procedures. The diagnosis of delayed perforation is reached using enhanced computed tomography, which was performed for patients with abdominal pain. Delayed bleeding was defined in patients who required endoscopic hemostasis or transfusion after ER.
All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS 22.0 Package; SPSS Inc., Chicago, Illinois, United States). Continuous variables are expressed as the means and were analyzed using Student’s t test. Categorical variables were compared with a χ2 test or, if appropriate, Fisher’s exact test. A probability value of < 5% was considered statistically significant.
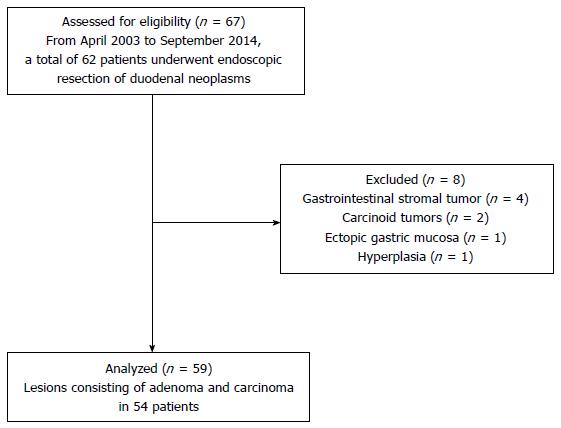
From April 2003 to September 2014, a total of 62 patients underwent ER of duodenal tumors. Four cases with gastrointestinal stromal tumors, two cases with carcinoid tumors, one case with an ectopic gastric mucosa, and one case with a hyperplasia were excluded. As a result, 59 lesions due to adenoma and carcinoma in 54 patients were analyzed (Figure 1).
The 59 cases included 39 males and 20 females. The average age was 61.3 years (range 40-79 years). Thirty-eight lesions were diagnosed as adenoma, and 21 lesions were diagnosed as carcinoma. The accuracy of the preoperative biopsy was 96.6% (57/59). Thirty-five lesions were located in the pre-ampulla region, and 24 were in the post-ampulla region. The average tumor size was 14.2 mm (95%CI: 11.6-16.8 mm). The macroscopic types included 12 polyploid and 47 superficial tumors. All lesions were confined to the mucosa. Thirty-six lesions underwent EMR. Piecemeal EMR was performed in four cases, and en-bloc EMR was performed in 32 cases. Among the piecemeal EMR cases, three lesions were removed in two pieces, and one lesion was removed in four pieces. Twenty-three lesions underwent ESD. Prophylactic clipping was applied in 46 patients.
Complications included perforation and bleeding (Table 1). Perforation occurred in eight (13%) patients (95%CI: 4.7%-22.6%). Four lesions were located in the pre-ampulla region, and four lesions were in the post-ampulla region. The mean size of lesion in cases of perforation was 22.9 mm, which was significantly different from the non-perforated group (P < 0.05). Intraoperative perforation occurred in six cases, and delayed perforation occurred two cases. Intraoperative perforation occurred in two EMR cases and ESD four cases. All cases in the EMR group and one case in the ESD group underwent conservative management after clipping. Three ESD cases required surgical management because they could not be repaired by clipping. Delayed perforation occurred in two ESD cases, and these patients required surgical management, even though both patients received prophylactic clipping. Perforation after ER was significantly associated with tumor size greater than 20 mm and ESD (Table 2). Bleeding occurred in two (3.4%) cases. One required endoscopic hemostasis, and the other patient received a transfusion after ER.
| Case | Age (yr) | Sex | Method | Complication | Clipping | Treatment | Hospital stay after ER (d) | Tumor characteristics | ||
| Location | Size (mm) | Type | ||||||||
| 1 | 65 | M | EMR | IP | Possible | Conservative | 7 | Post-ampulla | 17 | Is |
| 2 | 60 | M | EMR | IP | Possible | Conservative | 6 | Post-ampulla | 9 | IIa |
| 3 | 55 | M | ESD | DP | Possible | Surgical | 12 | Post-ampulla | 24 | IIa |
| 4 | 60 | M | ESD | Bleeding | Possible | Transfusion | 9 | Pre-ampulla | 20 | IIa |
| 5 | 67 | M | EMR | Bleeding | Possible | Hemostasis | 11 | Pre-ampulla | 55 | Isp |
| 6 | 40 | M | ESD | IP | Impossible | Surgical | 11 | Pre-ampulla | 20 | IIa |
| 7 | 55 | M | ESD | IP | Possible | Conservative | 18 | Pre-ampulla | 13 | IIc |
| 8 | 64 | M | ESD | IP | Impossible | Surgical | 16 | Post-ampulla | 30 | IIa |
| 9 | 44 | F | ESD | IP | Impossible | Surgical | 12 | Pre-ampulla | 30 | IIa |
| 10 | 72 | F | ESD | DP | Possible | Surgical | 15 | Pre-ampulla | 40 | IIa |
| Perforation | P value | |||
| Did not occur | Occurred | |||
| Sex | M | 33 | 6 | |
| F | 18 | 2 | 0.704 | |
| Histological diagnosis | Adenoma | 33 | 5 | |
| Carcinoma | 18 | 3 | 1.000 | |
| Tumor location | Pre-ampulla | 31 | 4 | |
| Post-ampulla | 20 | 4 | 0.704 | |
| Tumor size | Under 20 mm | 42 | 3 | |
| Over 20 mm | 9 | 5 | 0.014 | |
| Macroscopic type | Polyploid | 11 | 1 | |
| Superficial | 40 | 7 | 0.482 | |
| Resection method | EMR | 34 | 2 | |
| ESD | 17 | 6 | 0.047 | |
| Prophylactic clipping1 | Not applied | 10 | 0 | |
| Applied | 41 | 5 | 1.000 | |
For the surgical procedures, three cases consisted of suturing and covering with omentum. Two patients underwent Billroth I anastomosis after pyloric ring resection and partial duodenum resection. No patients with perforation who required surgery had postoperative complications. The patients were discharged at an average of 13.2 d after ER.
The reported incidence of malignant degeneration of duodenal tubulovillous polyps ranges from 35% to 85%, and accurately differentiating cancer from adenoma is difficult based on biopsy findings alone[8]. Even if the histopathological examination of a biopsy specimen reveals an adenoma, it is possible to diagnose an adenoma as carcinoma after ER. In our study, the accuracy of preoperative biopsy was 96.6% (57/59). An ER should be performed if no metastasis is present in the lymph nodes and distant organs; however, an adenoma in the duodenum presents the possibility of carcinoma. Nagatani et al[9] reported that the incidence of lymph node metastasis was 0% in cases of intramucosal cancer and 5% in cases of submucosal cancer. Shinoda et al[10] reported no cases of lymph node metastasis among 273 cases of early duodenal cancer. Therefore, an early duodenal neoplasm can be treated by ER, unless lymph node metastasis is revealed.
Some reports address ER for duodenal tumors, but none address standard therapy. The surgical methods include piecemeal EMR, en-bloc EMR, and ESD. Piecemeal EMR is possible in most tumors that exceed 20 mm, but commonly results in recurrence[11,12]. En-bloc EMR can be performed for tumors exceeding 10 mm, although the resection margins may be histologically positive[8]. Additionally, lesions larger than 20 mm cannot be safely removed en-bloc and closed by any currently available method[4,6,13]. Therefore, EMR is not an ideal treatment for duodenal neoplasms larger than 20 mm. ESD can be performed for tumors exceeding 20 mm and achieves higher rates of en-bloc and curative resection than EMR[5]. In one study, the negative margin rate was 100% for the lateral resection margin in ESD[8]. However, ESD is associated with a higher rate of complications, such as perforation and bleeding, than EMR[5]. Jung et al[14] reported that the perforation rates after ESD were very high (35.7%). For example, perforation rates associated with gastric ESD have been reported to be between 1.2% and 8.7%. Inoue et al[7] reported that the incidence of delayed perforation was significantly associated with post-ampullary tumor location and resection method (both piecemeal EMR and ESD). In our study, ER of tumors exceeding 20 mm and ESD presented a high risk of perforation. We examined EMR and ESD because piecemeal EMR was only performed in four cases, and therefore the statistical power was insufficient. Additionally, the results were not significantly different according to the tumor location.
As described earlier, ER of a duodenal tumor tends to cause complications (especially perforation), and appropriate treatments for perforation are lacking. Abundant blood vessels in the submucosal layer and a thin muscle layer in the duodenum are thought to be related to a high risk of complications. In addition, exposure of the duodenal wall to pancreatic juice and bile may increase the risk of delayed perforation[5]. Taku et al[15] reported that conservative treatment is possible when patients with perforation are stable. Krishna et al[16] reported that if perforation is suspected, abdominal CT should be performed to evaluate the indication for surgery. We have suggested that patients could be evaluated immediately by abdominal CT and receive emergency surgery, if necessary, when abdominal pain or high fever is present.
Prophylactic clipping is not sufficient to prevent perforation after ESD. Recently, a new device (the over-the-scope clip) has been developed for the prevention of perforation after ER, but this method requires further evaluation[17]. We suggest that laparoscopic and endoscopic cooperative surgery (LECS) should be the therapeutic strategy for tumors exceeding 20 mm.
Toyonaga et al[18] reported the use of an endo linear stapler for wedge resection. However, it is not possible to appropriately resect tumors of the posterior duodenum using this method (i.e., resection with an inappropriate margin or unnecessary resection of the duodenal wall)[18,19]. Sato et al[20] reported LECS of a duodenal carcinoid tumor. Recently, others have reported laparoscopic local excision of a tumor followed by closure of the defect using a hand-sewn technique[21-25]. We performed endoscopic total layer resection or ESD of a duodenal tumor followed by this procedure in three cases. All patients had no complications and were discharged in approximately one week. More cases should be evaluated in the future because the sample size of duodenal neoplasms was relatively small.
In conclusion, ESD for a duodenal tumor exceeding 20 mm may be associated with complications (especially perforation). ESD alone is not recommended for tumor treatment, and LECS should be considered as an alternative.
Duodenal neoplasms are relatively rare. Primary adenocarcinoma represents only 0.3% of all gastrointestinal tract malignant neoplasms and 0.042% of all malignant neoplasms. Therefore, no method of treatment for duodenal neoplasm has been established.
Recently, cases of endoscopic resection (ER) for superficial neoplasms without lymph node metastasis have been reported. ER may consist of endoscopic mucosal resection or endoscopic submucosal dissection (ESD). However, ER for the duodenum poses a high risk of complications. Patients with perforation undergo emergency surgery in many cases. It is unclear whether ER for duodenal tumors is appropriate.
The authors investigated predictors of perforation after ER for duodenal neoplasms without a papillary portion.
ESD for a duodenal tumor exceeding 20 mm may be associated with complications (especially perforation). ESD alone is not recommended for tumor treatment, and LECS should be considered as an alternative.
This paper presents an unique comparison of endoscopic mucosal dissection with endoscopic submucosal dissection in the management of non-ampullary duodenal tumours.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dumitrascu DL, Tovey FI S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Moss WM, McCart PM, Juler G, Miller DR. Primary adenocarcinoma of the duodenum. Arch Surg. 1974;108:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Spira IA, Ghazi A, Wolff WI. Primary adenocarcinoma of the duodenum. Cancer. 1977;39:1721-1726. [PubMed] [DOI] [Full Text] |
| 4. | Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc. 2009;69:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Fanning SB, Bourke MJ, Williams SJ, Chung A, Kariyawasam VC. Giant laterally spreading tumors of the duodenum: endoscopic resection outcomes, limitations, and caveats. Gastrointest Endosc. 2012;75:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Inoue T, Uedo N, Yamashina T, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Tatsuta M. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Endo M, Abiko Y, Oana S, Kudara N, Chiba T, Suzuki K, Koizuka H, Uesugi N, Sugai T. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Nagatani K, Takkekoshi T, Baba Y, Kaku S, Koizumi K, Fujii A, Ogata E, Ohta H, Nishi M, Kato Y. Indications for endoscopic treatment of early duodenal cancer: Based on cases reported in the literature. Endosc Digest. 1993;7:969–976. |
| 10. | Shinoda M, Makino A, Wada M, Kabeshima Y, Takahashi T, Kawakubo H, Shito M, Sugiura H, Omori T. Successful endoscopic submucosal dissection for mucosal cancer of the duodenum. Dig Endosc. 2010;22:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Jung JH, Choi KD, Ahn JY, Lee JH, Jung HY, Choi KS, Lee GH, Song HJ, Kim DH, Kim MY. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S. Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Krishna RP, Singh RK, Behari A, Kumar A, Saxena R, Kapoor VK. Post-endoscopic retrograde cholangiopancreatography perforation managed by surgery or percutaneous drainage. Surg Today. 2011;41:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Gubler C, Bauerfeind P. Endoscopic closure of iatrogenic gastrointestinal tract perforations with the over-the-scope clip. Digestion. 2012;85:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Toyonaga T, Nakamura K, Araki Y, Shimura H, Tanaka M. Laparoscopic treatment of duodenal carcinoid tumor. Wedge resection of the duodenal bulb under endoscopic control. Surg Endosc. 1998;12:1085-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Matsui H, Okamoto Y, Ishii A, Ishizu K, Kondoh Y, Aoki J, Yamazaki H, Ogoshi K, Makuuchi H. Endoscopy-assisted totally laparoscopic resection of a submucosal tumor of the duodenum. Tokai J Exp Clin Med. 2008;33:100-104. [PubMed] |
| 20. | Sato T, Fukunaga T, Ohyama S, Ueno M, Oya M, Yamamoto J, Saiura A, Yamaguchi T, Muto T, Kato Y. Endoscopic total layer resection with laparoscopic sentinel node dissection and defect closure for duodenal carcinoid. Hepatogastroenterology. 2005;52:678-679. [PubMed] |
| 21. | Yi NJ, Kim YW, Han HS, Fleischer GD. Duodenal polypectomy of Brunner’s gland hyperplasia using a novel laparoscopic technique. A case report. Surg Endosc. 2002;16:1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Lee JH, Han HS, Kim YW, Min SK, Lee HK. Laparoscopic wedge resection with handsewn closure for gastroduodenal tumors. J Laparoendosc Adv Surg Tech A. 2003;13:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Bowers SP, Smith CD. Laparoscopic resection of posterior duodenal bulb carcinoid tumor. Am Surg. 2003;69:792-795. [PubMed] |
| 24. | Orsenigo E, Di Palo S, Vignali A, Staudacher C. Laparoscopic excision of duodenal schwannoma. Surg Endosc. 2007;21:1454-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Kyuno D, Ohno K, Katsuki S, Fujita T, Konno A, Murakami T, Waga E, Takanashi K, Kitaoka K, Komatsu Y. Laparoscopic-endoscopic cooperative surgery is a safe and effective treatment for superficial nonampullary duodenal tumors. Asian J Endosc Surg. 2015;8:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |