Published online Apr 27, 2017. doi: 10.4240/wjgs.v9.i4.103
Peer-review started: November 23, 2016
First decision: December 29, 2016
Revised: January 28, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: April 27, 2017
Processing time: 157 Days and 12.5 Hours
To review surgical outcomes for patients undergoing pancreatectomy after proton therapy with concomitant capecitabine for initially unresectable pancreatic adenocarcinoma.
From April 2010 to September 2013, 15 patients with initially unresectable pancreatic cancer were treated with proton therapy with concomitant capecitabine at 1000 mg orally twice daily. All patients received 59.40 Gy (RBE) to the gross disease and 1 patient received 50.40 Gy (RBE) to high-risk nodal targets. There were no treatment interruptions and no chemotherapy dose reductions. Six patients achieved a radiographic response sufficient to justify surgical exploration, of whom 1 was identified as having intraperitoneal dissemination at the time of surgery and the planned pancreatectomy was aborted. Five patients underwent resection. Procedures included: Laparoscopic standard pancreaticoduodenectomy (n = 3), open pyloris-sparing pancreaticoduodenectomy (n = 1), and open distal pancreatectomy with irreversible electroporation (IRE) of a pancreatic head mass (n = 1).
The median patient age was 60 years (range, 51-67). The median duration of surgery was 419 min (range, 290-484), with a median estimated blood loss of 850 cm3 (range, 300-2000), median ICU stay of 1 d (range, 0-2), and median hospital stay of 10 d (range, 5-14). Three patients were re-admitted to a hospital within 30 d after discharge for wound infection (n = 1), delayed gastric emptying (n = 1), and ischemic gastritis (n = 1). Two patients underwent R0 resections and demonstrated minimal residual disease in the final pathology specimen. One patient, after negative pancreatic head biopsies, underwent IRE followed by distal pancreatectomy with no tumor seen in the specimen. Two patients underwent R2 resections. Only 1 patient demonstrated ultimate local progression at the primary site. Median survival for the 5 resected patients was 24 mo (range, 10-30).
Pancreatic resection for patients with initially unresectable cancers is feasible after high-dose [59.4 Gy (RBE)] proton radiotherapy with a high rate of local control, acceptable surgical morbidity, and a median survival of 24 mo.
Core tip: Patients undergoing pancreatectomy for resectable pancreas cancers have a significant risk of local and regional recurrence. That risk could be reduced if patients received moderate-dose preoperative radiotherapy. Many surgeons, however, are concerned that conventional X-ray-based radiotherapy could complicate what is already a complicated operation. The current series documents the surgical outcomes for 15 patients with initially unresectable pancreatic cancers who underwent pancreatectomy after high-dose [59.40 Gy (RBE)] proton-based radiotherapy. The lack of increased surgical toxicity suggests that proton radiotherapy may represent an optimal vehicle for the delivery of moderate dose neoadjuvant radiotherapy in the setting of resectable disease.
- Citation: Hitchcock KE, Nichols RC, Morris CG, Bose D, Hughes SJ, Stauffer JA, Celinski SA, Johnson EA, Zaiden RA, Mendenhall NP, Rutenberg MS. Feasibility of pancreatectomy following high-dose proton therapy for unresectable pancreatic cancer. World J Gastrointest Surg 2017; 9(4): 103-108
- URL: https://www.wjgnet.com/1948-9366/full/v9/i4/103.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i4.103
Patients undergoing pancreatectomy for tumors which are believed to be resectable by preoperative imaging experience high rates of lymph node positivity, margin positivity and local/regional recurrence[1-6]. In spite of this, many surgeons are reluctant to recommend neoadjuvant radiotherapy which might have the potential to sterilize microscopic disease in the operative bed and reduce the incidence of these events. This reluctance is presumably due to concerns that even moderate dose radiotherapy in the range of 50.40 Gy might complicate what is already a lengthy and complicated operation.
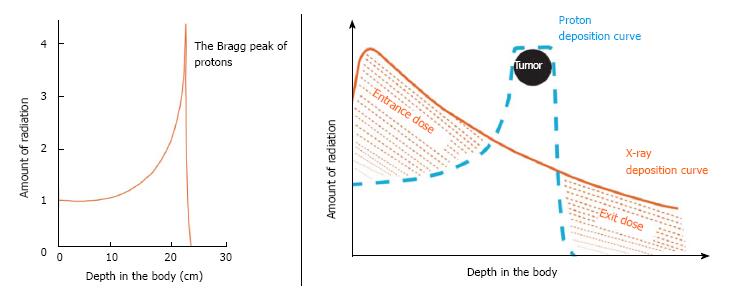
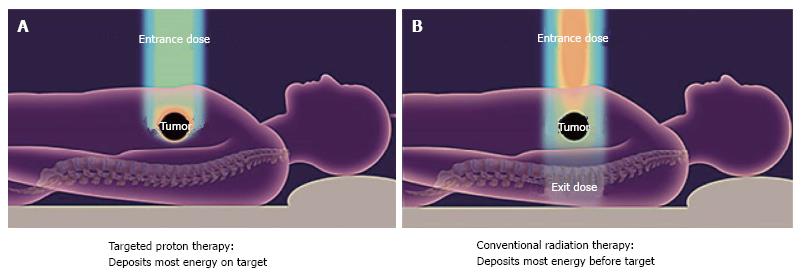
The current series reviews the surgical outcomes for a group of patients with initially unresectable disease who, after high-dose proton radiotherapy [59.40 Gy (RBE)] and chemotherapy (oral capecitabine, 1000 mg, twice a day), achieved enough of a radiographic response to justify surgical exploration. The favorable physical characteristics of proton radiotherapy are demonstrated in Figures 1 and 2. Specific attention is paid to the surgical metrics of: Duration of surgery; estimated blood loss; and hospital length of stay which are compared to benchmark studies in the surgical literature.
This is a retrospective single-institution study of patients enrolled on either the University of Florida Health Proton Therapy Institute PC-O1 trial for patients with unresectable disease or the University of Florida Health Proton Therapy Institute outcomes-tracking study. The statistical methods of this study were reviewed by Christopher G Morris from the Department of Radiation Oncology, University of Florida College of Medicine.
From April 20, 2010 to September 30, 2013, 15 patients with initially unresectable pancreatic cancer were treated with full-dose proton therapy with concomitant capecitabine at 1000 mg taken orally twice a day. All patients received 59.40 Gy (RBE) to the gross disease, and 1 patient also received 50.40 Gy (RBE) to the high-risk nodal targets. There were no treatment interruptions or chemotherapy dose reductions. Patient details can be found in Table 1.
| Patient | 1 | 2 | 3 | 4 | 5 |
| Age | 55 | 60 | 51 | 68 | 67 |
| Stage | T3 N1 | T4 N0 | T4 N0 | T4 N0 | T4 N0 |
| Comorbidities | None | Colon cancer | Unintentional weight loss | None | Unintentional weight loss |
| Resection type | Laparoscopic | Laparoscopic | Laparoscopic | Open | Open |
| Surgery duration (min) | 339 | 465 | 419 | 290 | 484 |
| Estimated blood loss (mL) | 300 | 800 | 850 | 2000 | 1000 |
| Intensive care stay (d) | 1 | 1 | 0 | 2 | 0 |
| Total hospital stay (d) | 5 | 11 | 6 | 10 | 14 |
| Complications | Wound infection | Delayed gastric emptying | None | None | Delayed gastric emptying and gastritis |
| Readmission within 30 d | 4 d for wound infection | 2 d for nausea and vomiting | None | None | 2 d for gastritis |
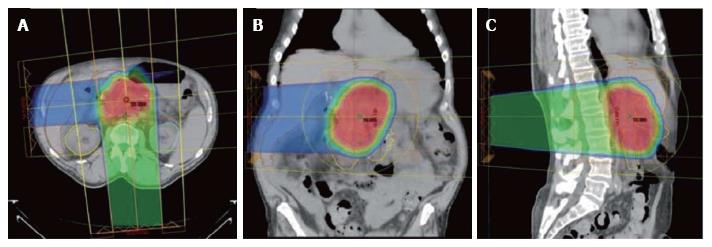
The technical details for the delivery of proton radiation therapy have been described previously[7,8]. In summary, optimized 2- or 3-field 3-dimensional conformal passive-scatter proton plans were created in which 95% of the planning target volumes received 100% of the prescribed dose, and 100% of the planning target volumes received at least 95% of the prescribed dose. Normal-tissue constraints included the following: Spinal cord, < 46 Gy; right kidney, V18 < 70%; left kidney, V18 < 30%; liver, V30 < 60%; and small bowel (including duodenum) and stomach, V20 < 50%, V45 < 15%, V50 < 10%, and V54 < 5%. These target coverage goals and normal-tissue limits were met for all patients with minor patient-specific adjustments. A typical proton therapy plan is shown in Figure 3.
To document surgical outcomes, we used treatment records to verify the type and extent of resection, procedure duration, blood volume lost during the procedure, length of hospital stay, number of days spent in intensive care, readmission for surgical complications, pathologic assessment of the surgical specimens, local disease control, distant disease control, and overall survival.
Six patients achieved a radiographic response sufficient to justify surgical exploration. Of these, 1 patient was identified as having intraperitoneal dissemination at the time of surgery and the planned pancreatectomy was aborted. Five patients underwent resection. Procedures included laparoscopic standard pancreaticoduodenectomy (n = 3), open pyloris-sparing pancreaticoduodenectomy (n = 1), and open distal pancreatectomy with irreversible electroporation of a pancreatic head mass (n = 1). Median age was 60 years (range, 51-67). These patients had been initially designated as having unresectable disease based on superior mesenteric artery and celiac artery encasement (n = 2), inferior vena cava encasement with invasion of the posterior abdominal wall (n = 1), biopsy-positive regional nodal metastasis (n = 1), or mesenteric root involvement with abutment of the celiac and hepatic arteries (n = 1).
Two patients underwent gross total (R0) resections and subsequent pathology showed minimal residual disease. Two patients had gross subtotal (R2) resections. One patient, who after negative pancreatic head biopsies underwent distal pancreatectomy and irreversible electroporation of the pancreatic head mass, had no identifiable malignancy in the surgical specimen. In none of the 5 cases did the surgeon document any complaint regarding the texture of tissue around the resection, exceptional bleeding, difficulty with closure, postoperative wound complications, or any other issue that could be attributed to the irradiated state of the tumor and surrounding tissues.
The median duration of the surgical procedures was 419 min (range, 290-484 min). Estimated blood loss (EBL) ranged from 300 to 2000 cm3 with a median of 850 cm3. The median intensive-care stay for these patients was one day (range, 0-2) and median hospital stay (LOS) was 10 d (range, 5-14). Three patients were readmitted to a hospital within 30 d after discharge: The first was a patient discharged on postoperative day 5 who was then readmitted for wound infection on day 9. The second was discharged on postoperative day 11 who was then readmitted the next day with the primary complaint of delayed gastric emptying. The third was a readmission on postoperative day 19 for ischemic gastritis following discharge on postoperative day 10.
Only 1 patient demonstrated ultimate local progression at the primary site, which occurred 7 mo after surgery in 1 of the patients who underwent an R0 resection. The median survival for the 5 resected patients was 24 mo (range, 10-30); the 4 patients with locally controlled disease ultimately developed distant metastases.
The above surgical metrics for patients with initially unresectable disease who received dose escalated radiotherapy to 59.4 Gy (RBE) compare favorably to those observed in four published studies that, for the most part, involved surgery for resectable patients who had not received neoadjuvant radiotherapy (Table 2): (1) Tseng et al[9] published a series analyzing 650 procedures performed by experienced surgeons at the MD Anderson Cancer Center (Houston, TX). The mean operative time was 513 min. The mean EBL was 725 cc and the average LOS was 13 d. The authors acknowledge that some patients underwent preoperative radiation therapy or chemotherapy but these numbers were not reported; (2) Speicher et al[10] reported an average procedure length of 431 min in a series of 140 pancreaticoduodenectomies performed by experienced surgeons in which 40% were performed laparoscopically. Patients experienced a mean EBL of 200 mL when a laparoscopic approach was used, and 500 mL with hybrid or open procedures. The mean LOS was 10 d with a 37% readmission rate. There is no mention of neoadjuvant therapy in these cases; (3) Asbun and Stauffer[11] at the Mayo Clinic reported similar metrics. For 215 open and 53 laparoscopic pancreaticoduodenectomies, the EBL averaged 1032 cm3 and 195 cm3, mean LOS was 12.4 d and 8 d, and average operating room time was 401 and 541 min, respectively. The authors did not record whether these patients had been irradiated before surgery; and (4) The Florida Agency for Healthcare Administration database[12] reported the statewide median length of stay following pancreatectomy in the years from 2010 to 2012 to be 11 d (mean ± SD, 14 ± 11.5).
| Published study | Operating room time (min) | Estimated blood loss (cc) | Length of hospital stay (d) |
| Tseng[9] | 513 | 725 | 13 |
| Speicher[10] open | NA | 500 | NA |
| Speicher[10] laparoscopic | NA | 200 | NA |
| Speicher[10] total | 431 | NA | 10 |
| Asbun[11] open | 401 | 1032 | 12.4 |
| Asbun[11] laparoscopic | 541 | 195 | 8 |
| Florida Agency for Healthcare Administration | NA | NA | 11 |
| Current series | 419 | 850 | 10 |
It is an accepted precept of oncology that patients with solid tumors cannot be cured if local and regional tumor control cannot be achieved. For patients with nonmetastatic pancreatic cancer, it is also generally accepted that local control cannot be achieved without extirpative surgery. As such, surgery represents a necessary condition for cure. Nevertheless, because surgery alone is associated with a high local and regional failure rate, it is rarely a sufficient condition for cure. Patients undergoing pancreaticoduodenectomy with negative lymph nodes and negative surgical margins will experience a 50%-80% chance of local-regional tumor recurrence if adjuvant therapies are not offered[1,2]. Even when postoperative chemotherapy and radiotherapy are delivered, the local-regional failure rates range from 28% in the Radiation Therapy Oncology Group 97-04 trial[3] to 36% in the Massachusetts General Hospital (Boston, MA) experience[4]. Although its methodological and statistical flaws have been well-described[13], the results of the European Study Group for Pancreatic Cancer-1 trial suggest that postoperative X-ray-based radiation therapy not only fails to improve patient survival but may be associated with a nominal survival decrement, presumably due to radiation therapy toxicity[14,15].
The failure of postoperative radiation therapy to even reliably sterilize microscopic disease in the postoperative setting might be explained in two ways: First, to allow for postoperative recovery after pancreaticoduodenectomy, upper abdominal radiation therapy cannot be delivered until 10 or 12 wk have elapsed. This time interval potentially allows for the progression of malignant cells in a hypoxic tumor bed. Second, because a large volume of small bowel is transposed into the postoperative radiation therapy field, it is generally not possible to deliver X-ray doses over 50 Gy, which may be inadequate to eradicate even microscopic disease growing in such a hypoxic environment.
While it is recognized that patients undergoing pancreatic resection have a high local-regional failure rate-even in the setting of negative surgical margins and negative lymph nodes-contemporary data from two high-volume institutions suggest that margin-negative, lymph node-negative pancreatectomies are relatively uncommon. The series published by investigators at Johns Hopkins University (Baltimore, MD) on 905 patients undergoing pancreaticoduodenectomy between 1995 and 2005 indicated a 41% margin-positivity rate and a 79% node-positivity rate[5]. The series from investigators at Memorial Sloan-Kettering Cancer Center (New York, NY) on 625 resections conducted between 2000 and 2009 indicated a 16% margin-positivity rate and a 70% node-positivity rate[6]. Based on these data, as well as the low likelihood of reliably sterilizing microscopic disease in the postoperative tumor bed with radiotherapy, it is likely that even “resectable” patients could benefit from preoperative radiation therapy, perhaps with fields that could cover regional lymph nodes. With the current series showing no increase in surgical morbidity after high dose proton radiotherapy, it is arguable that protons allow for the safe delivery of this oncologically rational intervention.
The surgical duration, EBL, and LOS for pancreatectomy following high-dose [59.40 Gy (RBE)] proton radiotherapy for patients with initially unresectable disease in this series are comparable to those observed in studies that, for the most part, involved surgery for resectable patients who had not received neoadjuvant radiotherapy. These data strongly suggest that standard dose [50.40 Gy (RBE)] neoadjuvant proton radiotherapy should not increase the difficulty of pancreatectomy in patients with resectable disease.
Nearly every patient cured of adenocarcinoma of the pancreas has had complete surgical resection of the tumor. Because this malignancy is initially asymptomatic, tumors are often very locally advanced at diagnosis and may not be resectable without removing vital tissues such as the major abdominal arteries. For many years chemotherapy and photon radiotherapy have been used to shrink advanced tumors in an attempt to make them resectable. Proton therapy has not previously been used for this purpose but is promising because it can be carefully shaped to spare the normal tissues of the abdomen such as the stomach, duodenum, spinal cord, and kidneys from radiation. This new treatment option will only be acceptable if it does not increase the rate of complications at the time of resection of the tumor.
Proton radiotherapy has been used in the treatment of cancer for many decades but has only recently become widely available. Much meticulous research must be done to show whether proton treatment offers advantages over standard treatments for each type of cancer. The first step in each line of inquiry is to demonstrate that proton radiotherapy is safe, and then efficacy can be addressed.
In the current work the authors have shown for the first time that proton radiotherapy given prior to attempted resection of initially unresectable pancreas cancers does not result in increased rates of surgical complications.
In the large fraction of patients with pancreatic cancer who have an unresectable tumor at the time of diagnosis, proton radiotherapy offers one safe option for neoadjuvant treatment intended to downstage the tumor and make surgical resection possible.
One patient in this study was treated with irreversible electroporation. This is an emerging technology in which the surgeon disrupts the integrity of tumor cell membranes using a high voltage, high frequency electrical field, leading to eventual cell death.
This paper is very interesting and suitable for publication in this journal.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Engelholm SA, Hotta T, Rausei S S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519-1524. [PubMed] |
| 3. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 4. | Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640-3650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Winter JM, Brennan MF, Tang LH, D’Angelica MI, Dematteo RP, Fong Y, Klimstra DS, Jarnagin WR, Allen PJ. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Sachsman S, Nichols RS, Morris CG, Zaiden R, Johnson EA, Awad Z, Bose D, Ho MW, Huh SN, Li Z. Proton Therapy and Concomitant Capecitabine for Non-Metastatic Unresectable Pancreatic Adenocarcinoma. Int J Particle Ther. 2014;1:692-701. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Nichols RC, Huh SN, Prado KL, Yi BY, Sharma NK, Ho MW, Hoppe BS, Mendenhall NP, Li Z, Regine WF. Protons offer reduced normal-tissue exposure for patients receiving postoperative radiotherapy for resected pancreatic head cancer. Int J Radiat Oncol Biol Phys. 2012;83:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, Sun CC, Evans DB. The learning curve in pancreatic surgery. Surgery. 2007;141:694-701. [PubMed] |
| 10. | Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG, Clary BM, Pappas TN, Tyler DS, Perez A. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol. 2014;21:4014-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Ryan CE, Wood TW, Ross SB, Smart AE, Sukharamwala PB, Rosemurgy AS. Pancreaticoduodenectomy in Florida: do 20-year trends document the salutary benefits of centralization of care? HPB (Oxford). 2015;17:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet. 2001;358:1565-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [PubMed] |
| 15. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1906] [Article Influence: 90.8] [Reference Citation Analysis (0)] |