Published online Mar 27, 2017. doi: 10.4240/wjgs.v9.i3.92
Peer-review started: August 26, 2016
First decision: September 27, 2016
Revised: November 12, 2016
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: March 27, 2017
Processing time: 211 Days and 12.8 Hours
We describe the case of a patient successfully reconstructed with laparoscopic retrosternal gastric pull-up after esophagectomy for unresectable posterior mediastinal inflammatory myofibroblastic tumor, eroding into the esophagus and compressing the airways. A partial esophagectomy with esophagostomy was performed for treatment of esophageal pleural fistula and empyema, while the airways were managed with the placement of an endobronchial stent. Gastrointestinal reconstruction was performed using a laparoscopic approach to create a retrosternal tunnel for gastric conduit pull-up and cervical anastomosis. The patient was discharged uneventfully after 6 d, and has done very well at home with normal diet.
Core tip: Retrosternal gastric tube has been used in various clinical scenarios, for both malignant and benign esophageal disease. The laparoscopic approach allowed for a simple, fast, and controlled dissection of the retrosternal plain and reconstruction of the alimentary tract. This approach should be considered as a valid alternative for reconstruction of the alimentary tract in patients where the prevertebral route is not available.
- Citation: Mungo B, Barbetta A, Lidor AO, Stem M, Molena D. Laparoscopic retrosternal gastric pull-up for fistulized mediastinal mass. World J Gastrointest Surg 2017; 9(3): 92-96
- URL: https://www.wjgnet.com/1948-9366/full/v9/i3/92.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i3.92
The safety and feasibility of laparoscopic retrosternal esophageal bypass using a gastric conduit has recently been described for the management of esophageal corrosive strictures[1]. Retrosternal gastric pull-up can be used for reconstruction after esophagectomy when a prevertebral route is not available, or as an option to create an esophageal bypass for unresectable esophageal tumors[2]. We report herein a successful case of totally laparoscopic retrosternal gastric pull-up for a fistulized unresectable mediastinal inflammatory myofibroblastic tumor.
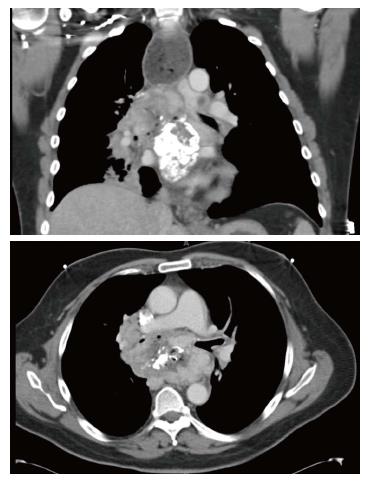
A 52-year-old man was referred to our division for evaluation of a mediastinal mass, initially detected two years earlier on a computed tomography performed for back pain. The patient was treated with steroids for presumptive fibrosing mediastinitis, however his symptoms progressively increased, with worsening shortness of breath, stridor and severe dysphagia. Multiple biopsies performed through esophagogastroduodenoscopy (EGD), bronchoscopy and mediastinoscopy failed to provide a diagnosis. The bronchoscopy showed extrinsic compression of the right main stem bronchus as well as the right upper lobe bronchus and the bronchus intermedius. The latter was almost completely obstructed and required placement of an endobronchial stent. EGD revealed a large mass eroding into the esophageal wall. A follow up computed tomography (CT) scan confirmed the presence of an enlarging subcarinal mass, measuring approximately 9.7 cm × 6 cm × 8.6 cm, completely surrounding the carina, the bronchi bilaterally, the esophagus and compressing the left atrium (Figure 1). A PET scan showed the mediastinal mass to be intensely hypermetabolic. After VATS biopsy of the mass, the patient developed empyema due to creation of esophageal-mass-pleural communication. Partial esophagectomy and infraclavicular esophagostomy was performed to treat the fistula and facilitate resolution of the empyema, although complete resection of the mass was not achievable. The final pathology of the resected specimen was consistent with inflammatory myofibroblastic tumor. The patient subsequently underwent treatment with high-dose steroids and definitive radiation. After appropriate recovery, he was admitted for gastrointestinal reconstruction. Due to unavailability of the prevertebral route, which was occupied by the unresectable mass, a retrosternal route was chosen.
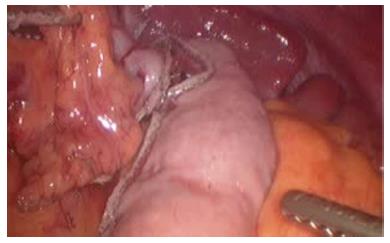
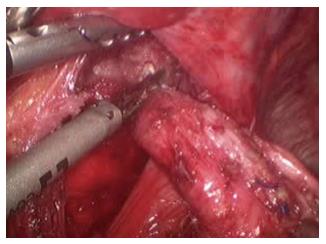
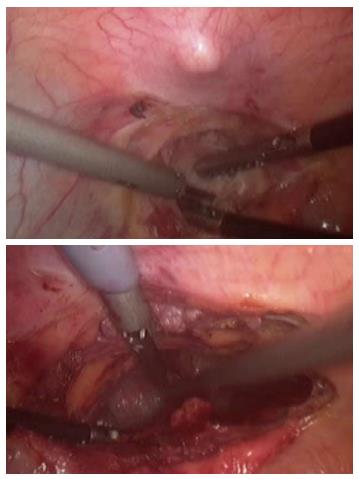
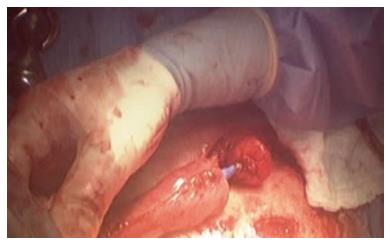
The patient was placed in supine position on the operating table and a standard laparoscopic approach was used. After complete mobilization of the stomach, a 10-cm wide gastric conduit was created by dividing the right and left gastric arteries and the proximal portion of the stomach (Figure 2). The distal esophageal stump was dissected free from the mediastinal attachments, removed en-bloc with the proximal stomach and sent to pathology with no residual tumor identified (Figure 3). Pyloric drainage was achieved via injection 200 units of botulin toxin into the pyloric muscle. The substernal dissection was then started immediately posterior to the xyphoid process (Figure 4). The avascular plane between the pericardium, the sternum and bilateral mediastinal pleura was developed with ultrasonic dissection and a tunnel about 15-cm wide was created from the abdomen all the way up to the thoracic inlet (Figure 4). The esophagostomy was then taken down and the proximal esophagus was exposed through a left cervical incision. The dissection from the neck was carried down to the substernal tunnel previously created. The gastric conduit was then pulled-up to the neck and the proximal portion of the stomach was externalized through the cervical incision. The conduit was very well perfused and the length was excellent. A stapled anastomosis using a 28-French circular mechanical stapler was performed (Figure 5) and the tip of the conduit was resected with a linear stapler. The anastomosis was pulled down below the sternal notch. The gastric conduit was secured to the diaphragm in order to avoid herniation of intra-abdominal organs into the mediastinum and a feeding jejunostomy was placed.
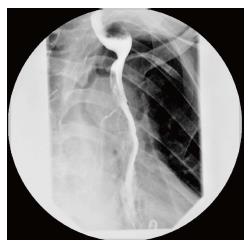
The patient had an uneventful recovery. A swallow study showed good gastric emptying and no anastomotic leak (Figure 6) and the patient was started on liquid diet and discharged on post-operative day 6. At home, he was gradually advanced to regular diet and weaned off tube feeding. At 8 mo after the procedure, he is eating a regular diet and has no symptoms.
Retrosternal gastric tube reconstruction has been used in various clinical scenarios, for both malignant and benign esophageal disease. Esophageal bypass surgery can be an option to treat patients with a fistula between the esophagus and the airways, providing relief from aspiration symptoms through separation of the respiratory and alimentary tracts[3]. Moreover, retrosternal gastric pull-up has been reported to be particularly beneficial for patients at high risk of developing locoregional recurrence after esophagectomy to prevent conduit obstruction and inability to eat[4]. In a randomized study by van Lanschot et al[4], retrosternal gastric tube reconstruction was described as a simple and safe technique with similar technical, functional results and postoperative recovery to a prevertebral reconstruction[5]. Retrosternal bypass can also be useful for unexpected unresectable cancers or perforated ones[5]. Javed et al[1,6] have recently described a laparoscopic technique for esophageal bypass in patients with corrosive esophageal strictures, using either a gastric or a colonic conduit. The authors reported on safety, feasibility and effectiveness of the minimally invasive technique, along with the well-known advantages, such as faster recovery and minimal postoperative pain. Moreover, the use of laparoscopy allowed direct visualization of the substernal dissection, avoiding injury to the pleura or lung and negligible blood loss[1,6].
An alternative route that can be used is the placement of the conduit in the subcutaneous space. This approach has several disadvantages: It is the longest route available, often needing either the colon or the jejunum as conduits, it is associated with higher risk of conduit trauma and twisting and has a potential negative esthetical impact. For these reasons the subcutaneous route is reserved as the last option for patients with previous mediastinal surgery and pleural infection or mediastinal fibrosis[7,8].
Esophageal reconstruction most commonly involves the stomach (< 90%)[9], followed by colon, jejunum, and pedicle skin-muscle flaps. Colon interposition is the first choice for selected patients with esophageal cancer when the stomach is unavailable or for benign esophageal diseases especially in young patients, with the intent of preserving the stomach[10]. Although long-term results for coloplasty are similar to gastroplasty, the reconstruction with the stomach generally involves a simpler operation with only one anastomosis[11]. The gastric conduit is usually better perfused than the colon leading to a lower incidence of conduit necrosis[12,13].
We found retrosternal pull-up a particularly well suited technique for the case we described, due to non-availability of prevertebral route, unresectability of the mediastinal mass and history of empyema. The laparoscopic approach allowed for a simple, fast, and controlled dissection of the retrosternal plain and reconstruction of the alimentary tract. This approach should be considered as a valid alternative for reconstruction of the alimentary tract in patients where the prevertebral route is not available.
A 52-year-old man presented with a 2-year history of mediastinal mass causing back pain, and increase of shortness of breath, stridor and severe dysphagia.
Mediastinal mass compressing the right main stem bronchus, right upper lobe and intermedius bronchus and eroding the esophageal wall.
Lung cancer, sarcoma, esophageal cancer.
Computed tomography scan showed an enlarging subcarinal mass, completely surrounding the carina, bronchi bilaterally the esophagus and compressing the left atrium. Positron emission computed tomography scan showed a hypermetabolic mass. Esophagogastroduodenoscopy and video-assisted thoracic surgery biopsy.
The resected specimen was consistent with inflammatory myofibroblastic tumor.
Partial esophagectomy and infraclavicular esophagostomy, high-dose of steroids and definitive radiation and a finally gastrointestinal reconstruction with a retrosternal route.
To treat esophageal-mass-pleural fistula and facilitate resolution of the empyema a partial esophagectomy with esophagostomy were performed, with no complete resection of mass achievable. The gastric tube was chosen for alimentary tract reconstruction. The retrosternal route was used as the prevertebral route was unavailable due to the unresectability of the mediastinal mass and history of empyema.
VATS: Video-assisted thoracic surgery. Inflammatory myofibroblastic tumor is a rare benign or locally aggressive tumor. It is characterized by dense inflammatory infiltrated cells in a myxoid or collagenous stroma.
The retrosternal gastric tube is a valid approach for reconstruction of alimentary tract when a prevertebral route is unsuitable. Furthermore this esophageal bypass represents an option to treat patients with airways-esophagus fistula. Minimally invasive approach provides different advantages such as a direct visualization during substernal dissection, as well as a fast recovery and minimal postoperative pain.
It’s a well described case of a laparoscopic retrosternal gastric bypass.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Contini S, Liakakos TK S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Javed A, Agarwal AK. Laparoscopic retrosternal bypass for corrosive stricture of the esophagus. Surg Endosc. 2012;26:3344-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 2. | Meunier B, Spiliopoulos Y, Stasik C, Lakéhal M, Malledant Y, Launois B. Retrosternal bypass operation for unresectable squamous cell cancer of the esophagus. Ann Thorac Surg. 1996;62:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Hihara J, Hamai Y, Emi M, Aoki Y, Taomoto J, Miyata Y, Okada M. Esophageal bypass operation prior to definitive chemoradiotherapy in advanced esophageal cancer with tracheobronchial invasion. Ann Thorac Surg. 2014;97:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | van Lanschot JJ, van Blankenstein M, Oei HY, Tilanus HW. Randomized comparison of prevertebral and retrosternal gastric tube reconstruction after resection of oesophageal carcinoma. Br J Surg. 1999;86:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Whooley BP, Law S, Murthy SC, Alexandrou A, Chu KM, Wong J. The Kirschner operation in unresectable esophageal cancer: current application. Arch Surg. 2002;137:1228-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Javed A, Agarwal AK. Total laparoscopic esophageal bypass using a colonic conduit for corrosive-induced esophageal stricture. Surg Endosc. 2013;27:3726-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Perez M, Haumont T, Arnoux JM, Redjaimia I, Rouard N, Blum A, Reibel N, Jay N, Braun M, Grosdidier G. Anatomically based comparison of the different transthoracic routes for colon ascension after total esogastrectomy. Surg Radiol Anat. 2010;32:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ferrer JM, Bruck HM. Jejunal and colonic interposition for non-malignant disease of the esophagus. Ann Surg. 1969;169:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Müller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 624] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Gust L, Ouattara M, Coosemans W, Nafteux P, Thomas PA, D’Journo XB. European perspective in Thoracic surgery-eso-coloplasty: when and how? J Thorac Dis. 2016;8:S387-S398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Urschel JD. Does the interponat affect outcome after esophagectomy for cancer? Dis Esophagus. 2001;14:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin. 2006;16:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg. 2003;138:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |