Published online Feb 27, 2017. doi: 10.4240/wjgs.v9.i2.53
Peer-review started: September 1, 2016
First decision: October 26, 2016
Revised: November 19, 2016
Accepted: December 16, 2016
Article in press: December 19, 2016
Published online: February 27, 2017
Processing time: 179 Days and 7.9 Hours
To assess nutritional recovery, particularly regarding feeding jejunostomy tube (FJT) utilization, following upper gastrointestinal resection for malignancy.
A retrospective review was performed of a prospectively-maintained database of adult patients who underwent esophagectomy or gastrectomy (subtotal or total) for cancer with curative intent, from January 2001 to June 2014. Patient demographics, the approach to esophagectomy, the extent of gastrectomy, FJT placement and utilization at discharge, administration of parenteral nutrition (PN), and complications were evaluated. All patients were followed for at least ninety days or until death.
The 287 patients underwent upper GI resection, comprised of 182 esophagectomy (n = 107 transhiatal, 58.7%; n = 56 Ivor-Lewis, 30.7%) and 105 gastrectomy [n = 63 subtotal (SG), 60.0%; n = 42 total (TG), 40.0%]. 181 of 182 esophagectomy patients underwent FJT, compared with 47 of 105 gastrectomy patients (99.5% vs 44.8%, P < 0.0001), of whom most had undergone TG (n = 39, 92.9% vs n = 8 SG, 12.9%, P < 0.0001). Median length of stay was similar between esophagectomy and gastrectomy groups (14.7 d vs 17.1 d, P = 0.076). Upon discharge, 87 esophagectomy patients (48.1%) were taking enteral feeds, with 53 (29.3%) fully and 34 (18.8%) partially dependent. Meanwhile, 20 of 39 TG patients (51.3%) were either fully (n = 3, 7.7%) or partially (n = 17, 43.6%) dependent on tube feeds, compared with 5 of 8 SG patients (10.6%), all of whom were partially dependent. Gastrectomy patients were significantly less likely to be fully dependent on tube feeds at discharge compared to esophagectomy patients (6.4% vs 29.3%, P = 0.0006). PN was administered despite FJT placement more often following gastrectomy than esophagectomy (n = 11, 23.4% vs n = 7, 3.9%, P = 0.0001). FJT-specific complications requiring reoperation within 30 d of resection occurred more commonly in the gastrectomy group (n = 6), all after TG, compared to 1 esophagectomy patient (12.8% vs 0.6%, P = 0.0003). Six of 7 patients (85.7%) who experienced tube-related complications required PN.
Nutritional recovery following esophagectomy and gastrectomy is distinct. Operations are associated with unique complication profiles. Nutritional supplementation alternative to jejunostomy should be considered in particular scenarios.
Core tip: Adequate nutrition following major upper gastrointestinal cancer resection is critical in order to achieve optimal recovery. However, feeding jejunostomy tube placement should not be considered obligatory as part of upper gastrointestinal resection. Alternative methods of nutritional supplementation are available and perhaps better-tolerated.
- Citation: Blakely AM, Ajmal S, Sargent RE, Ng TT, Miner TJ. Critical analysis of feeding jejunostomy following resection of upper gastrointestinal malignancies. World J Gastrointest Surg 2017; 9(2): 53-60
- URL: https://www.wjgnet.com/1948-9366/full/v9/i2/53.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i2.53
Upper gastrointestinal malignancy, comprised of esophageal and gastric cancer, represents nearly 42000 new diagnoses per year in the United States. These diagnoses carry a high disease-related mortality, causing an estimated 26000 deaths annually[1]. Patients with esophageal and gastric malignancies often present in a malnourished state, with significant unintentional weight loss a common sign of disease. Such weight loss has been associated with worse outcomes following resection[2]. Adequate nutrition for patients undergoing resection is critical in order to recover from the operation and to successfully undergo adjuvant therapy.
Nutritional support modalities include enteral nutrition via feeding tubes and parenteral nutrition (PN) via central venous catheters. Enteral feeding is preferred as it has been shown to maintain the epithelial lining of the gut in animals, with limited evidence of the same in humans[3,4]. However, enterally-fed patients are often unable to meet prescribed caloric goals due to postoperative dysmotility, tube malfunctions, missed feedings, or other reasons[5,6]. Parenteral nutrition has been used postoperatively when patients demonstrate that they are unable to orally or enterally achieve adequate caloric intake, with the benefit of consistent nutritional support. However, parenteral nutrition has been associated with a higher incidence of infectious complications[7]. Regarding oncology patients, Bozzetti et al[8] randomized 317 patients undergoing major gastrointestinal cancer resection to either enteral or parenteral nutritional support immediately postoperatively, finding lower overall, and specifically infectious, complication rates in enterally-supported patients.
Options for nutritional support following upper gastrointestinal resection include needle catheter jejunostomy, Stamm or Witzel jejunostomy, or nasojejunal feeding tube placement[9-14]. In some centers, feeding jejunostomy (FJT) is routinely performed following esophagectomy or total gastrectomy, with more selective utilization with subtotal gastrectomy. However, other groups advocate selective use of FJT to minimize tube-related complications[15]. This study examined parenteral nutrition administration and feeding tube utilization rates at the time of discharge in order to better assess the need for enteral support following upper gastrointestinal resection.
The medical records for all patients who underwent esophagectomy and total or subtotal gastrectomy with curative intent from January 2001 to December 2014 were identified from a prospectively-maintained database. Patients’ demographic information, procedure performed, utilization of nutritional support, post-operative length of stay, and post-operative complications were obtained from the medical record. Surgical complications within 30 d after the operation were graded using a surgical secondary events grading system, as described elsewhere, in which grade 1 complications required local or bedside care; grade 2 complications required invasive monitoring or intravenous medication; grade 3 complications required an operation, interventional radiology procedure, intubation, or therapeutic endoscopy; grade 4 complications resulted in a persistent disability or required major organ resection; and grade 5 complications resulted in death[16].
Nutritional support was considered to have been utilized if the patient was not able to achieve adequate oral intake during hospital admission and therefore (1) received PN post-operatively while an inpatient and/or home PN at time of discharge or (2) required tube feeds to meet caloric goals at the time of discharge. PN was administered via triple-lumen subclavian or internal jugular venous lines or peripherally-inserted central catheters. Of note, all PN in our institution is managed by a physician-led multi-disciplinary team in conjunction with the primary service. All of the surgeons performing upper GI resections were observed by a second attending for a minimum of five cases to ensure technical uniformity and quality of feeding jejunostomy placement in order to confirm that the complications were not technical in nature. Jejunostomy was performed in conjunction with upper gastrointestinal resection in order to gain enteral access to (1) provide nutritional support in the immediate post-operative phase or (2) supplement caloric intake in the event that the patient could not meet nutritional goals with oral intake. Feeding jejunostomy-related complications were considered as such when an invasive intervention was required, such as interventional radiology procedure or reoperation; improper tube function such as clogging was not considered a complication.
Our institutional esophagectomy protocol is to keep the patient nil per os for seven days after resection, with nasogastric tube decompression of the conduit until post-operative day six. Trophic tube feeds are started on post-operative day two and slowly advanced to goal. Patients undergo thin barium swallow to evaluate for anastomotic leak on post-operative day seven, and if negative they are advanced first to clear liquids, then full liquids, and finally post-esophagectomy diet. If calorie counts demonstrate adequate intake, the patients are discharged without tube feeds. Tube feeds are continued on discharge if patients are unable to take oral diet or do not meet caloric requirements by mouth.
Our institutional subtotal gastrectomy protocol is to keep the patient nil per os with nasogastric tube decompression until the patient has return of bowel function. The tube is removed and the patient’s diet is advanced as tolerated from clear liquids to post-gastrectomy diet. The total gastrectomy protocol is to keep the patient nil per os with nasogastric tube decompression until they undergo diatrizoic acid swallow to evaluate for anastomotic leak, on post-operative day seven. If the study is negative, the nasogastric tube is removed and the patient is advanced first to clear liquids, then full liquids, and finally post-gastrectomy diet. Enteral feeds are started in patients who are unable to tolerate oral feedings within the seven to ten days following operations. If calorie counts demonstrate adequate intake, the patients are discharged without tube feeds. Tube feeds are continued on discharge if patients are unable to take oral diet or do not meet caloric requirements by mouth.
All patients meeting inclusion criteria were identified and followed up for a minimum of 180 d or until death. Data were analyzed using SAS statistical software, version 5.0 (SAS Institute, Inc., Cary, NC). Data were expressed as percentages in the case of categorical variables. Frequencies were compared by the χ2 test. Means of continuous variables were analyzed using t test or ANOVA. All reported P values were two-tailed and for all tests values less than 0.05 were considered significant. This study was approved by the institutional review board at Rhode Island Hospital.
Resection of an upper gastrointestinal malignancy was performed in 287 patients. The median patient age and proportion of males were similar between the esophagectomy and gastrectomy groups. There was no significant difference in mean length of stay groups (14.7 d vs 17.1 d, P = 0.076). Within the gastrectomy group, the median length of stay was significantly longer for the TG group compared to the SG group (16 d vs 10 d, P = 0.0002). Patients were more likely to be fully dependent on tube feeds at discharge following esophagectomy than gastrectomy (n = 53, 29.3% vs n = 3, 6.4%; P = 0.0006). Within 30 d of operation, 52.4% of TG and 29.6% of SG patients experienced complications, compared to 91 patients (50.0%) from the esophagectomy group. Major complications (grade 3-5) occurred in 59 esophagectomy patients and 26 gastrectomy patients (32.6% vs 24.8%, P = 0.18). Feeding tube-specific complications requiring reoperation within 30 d of operation occurred in 6 of 47 gastrectomy patients (12.8%), all within the TG group (P = 0.23). Complications were comprised of closed-loop obstruction around the feeding tube (n = 2), feeding tube leak (n = 2), small bowel perforation (n = 1), and multi-organ failure after initiation of tube feeds (n = 1). Conversely, within the esophagectomy group, only one jejunostomy tube-related major complication presented in follow-up, a small bowel obstruction at the jejunostomy site in a patient who had undergone transhiatal esophagectomy who required reoperation (Table 1).
| Complication | Subtotal gastrectomy (n = 63) | Total gastrectomy (n = 42) | Esophagectomy (n = 182) |
| None | 45 (71.4) | 20 (47.6) | 91 (50.0) |
| Low-grade | 10 (15.9) | 4 (9.5) | 32 (17.6) |
| High-grade | 8 (12.7) | 15 (35.7) | 54 (29.7) |
| Overall mortality | 0 (0.0) | 3 (7.1) | 5 (2.7) |
| Tube-related complications | 0 | 6 | 1 |
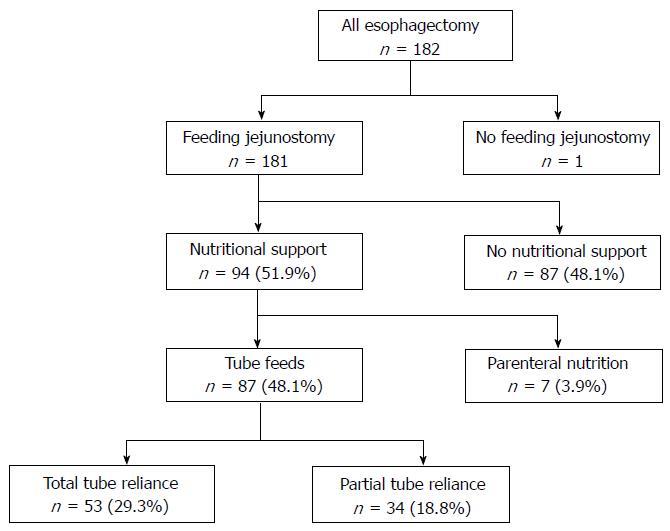
Between January 2001 and June 2014, 182 patients underwent esophagectomy for esophageal malignancy with curative intent (Figure 1). Patients’ median age was 64.0 years and 145 were male (79.7%). The predominant tumor type consisted of adenocarcinoma (n = 158, 86.8%), followed by squamous cell carcinoma (n = 15, 8.2%), high grade dysplasia (n = 8, 4.3%), and neuroendocrine tumor (n = 1, 0.5%). The primary tumor was located in the middle third of the esophagus in 11 patients (6.0%), lower third in 144 patients (79.1%), and at the gastroesophageal junction in 27 patients (14.8%). One hundred and seven patients (58.7%) underwent transhiatal esophagectomy, 56 patients (30.7%) had Ivor-Lewis esophagectomy, 10 patients (5.4%) underwent three-incision esophagectomy, and 9 patients (4.9%) had thoracoabdominal esophagectomy. Endoscopic ultrasound was used during pre-operative staging in 70 patients (38.4%). Neo-adjuvant induction therapy was administered to 114 patients (62.6%).
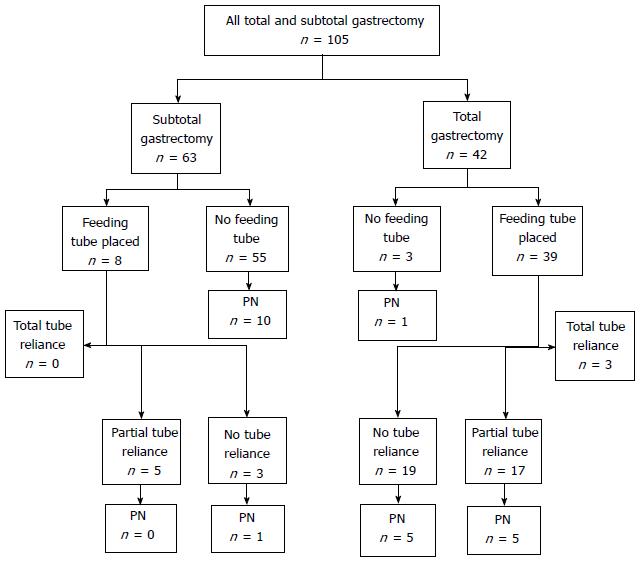
Between January 2004 and December 2013, 105 patients underwent total gastrectomy (TG) (n = 42, 40%) or subtotal gastrectomy (SG) (n = 63, 60%) (Figure 2). The TG and SG groups had similar proportions of males (66.7% each), however, the TG group was younger compared to the SG group (66.6 years vs 72.7 years, respectively, P = 0.018). Pre-operative albumin was obtained from the medical record in 36 TG patients (85.7%) and 41 SG patients (65.1%); mean albumin was higher in the TG group compared to the SG group (3.5 vs 3.2, P = 0.024).
A feeding jejunostomy tube was placed in 181 of the 182 esophagectomy patients (99.5%). At the time of discharge, 87 esophagectomy patients (48.1%) required tube feeds for nutritional supplementation, of whom 53 (29.3%) were fully and 34 (18.8%) were partially reliant (Table 2). There was no association between tube feed requirement and age, gender, tumor type, or administration of induction therapy. Patients who had undergone transhiatal esophagectomy were more likely to require tube feeds at discharge than patients who underwent Ivor-Lewis esophagectomy (64 of 107 transhiatal, 59.8% vs 14 of 56 Ivor-Lewis, 25.0%; P < 0.0001) (Table 3). Meanwhile, seven patients (3.9%) were discharged on parenteral nutrition, four for chylothorax and three having had the feeding tube removed on reoperation (for hemoperitoneum, evisceration, and anastomotic leak). Of the patients with transhiatal esophagectomy, 56 of 107 patients (52.3%) had a complication, of which 34 were cervical anastomotic leak (31.8%). Fifteen of 56 patients (26.8%) with Ivor-Lewis esophagectomy experienced complications, of which four were anastomotic leaks (7.1%). The difference in anastomotic leak rate between the two approaches was statistically significant (P = 0.0003).
| Characteristic | Total (n = 182) | Tube feeds used | Tube feeds not used | P value |
| Age > 65 yr | 93 | 40 (43.0) | 53 (57.0) | 0.24 |
| Male sex | 145 | 69 (47.6) | 76 (52.4) | 0.91 |
| Tumor type | ||||
| Adenocarcinoma | 158 | 76 (48.1) | 82 (51.9) | |
| Squamous cell carcinoma | 15 | 7 (46.7) | 8 (53.3) | 0.99 |
| High-grade dysplasia | 8 | 4 (50.0) | 4 (50.0) | |
| Neo-adjuvant therapy | 114 | 52 (45.6) | 62 (54.4) | 0.54 |
| Post-operative complication | 91 | 66 (72.5) | 25 (27.5) | < 0.0001 |
| Esophagectomy approach | ||||
| Transhiatal | 107 | 64 (59.8) | 43 (40.2) | < 0.0001 |
| Ivor-Lewis | 56 | 14 (25.0) | 42 (75.0) |
| Tube feed reliance | Transhiatal (n = 107) | Ivor-lewis (n = 56) | Other (n = 19) |
| None | 43 (40.2) | 42 (75.0) | 10 (52.6) |
| Partial | 20 (18.7) | 10 (17.6) | 4 (21.1) |
| Total | 44 (41.1) | 4 (7.1) | 5 (26.3) |
A feeding jejunostomy tube was placed for 47 of the 105 gastrectomy patients (44.8%), of which significantly more were performed for the TG than the SG group (92.9% vs 12.9%, P < 0.0001). After TG with feeding tube, 20 of 39 patients (51.3%) were fully (n = 3, 7.7%) or partially (n = 17, 43.6%) dependent on tube feeds at the time of discharge, whereas after SG with feeding tube, 5 of 8 (62.5%) were partially dependent and no patients were fully dependent on tube feeds (Table 4). Need for tube feed-based nutritional support in gastrectomy patients was not associated with extent of resection (51.3% TG vs 62.5% SG, P = 0.56). During admission, 11 TG and 11 SG patients (26.2% vs 17.4%, respectively) required PN as a bridge to adequate oral or enteral intake. Following TG with feeding tube placement, 10 of the 39 patients (25.6%) required PN, whereas one of the SG with feeding tube placement patients required PN. Three patients (2.9%) required home parenteral nutrition, of whom two had had tube-related complications and one had persistent feeding intolerance. For TG and SG patients, PN administration was not associated with extent of resection (11 of 42 TG, 26.2%, vs 11 of 63 SG, 17.5%; P = 0.28), feeding tube placement (11 of 47 with tube, 23.4% vs 11 of 58 without tube, 19.0%; P = 0.58), or feeding tube utilization (5 of 25 with tube utilization, 20.0% vs 6 of 22 without tube utilization, 27.3%; P = 0.56).
| Variable | Overall (n = 105) | Subtotal (n = 63) | Total (n = 42) | P value |
| Feeding tube placed | 47 (44.8) | 8 (12.7) | 39 (92.9) | < 0.0001 |
| Tube placed, utilized | 25 (53.2) | 5 (62.5) | 20 (51.3) | 0.71 |
| Tube placed, utilized, PN utilized | 5 (10.6) | - | 5 (12.8) | 0.57 |
| Tube placed, not utilized | 22 (46.8) | 3 (37.5) | 19 (48.7) | 0.71 |
| Tube placed, not utilized, PN utilized | 6 (12.8) | 1 (12.5) | 5 (12.8) | 1.0 |
| PN utilized | 22 (21.0) | 11 (17.5) | 11 (26.2) | 0.28 |
| PN utilized with feeding tube | 11 (10.5) | 1 (9.1) | 10 (90.9) | 0.42 |
| PN utilized without feeding tube | 11 (10.5) | 10 (90.9) | 1 (9.1) | 0.51 |
| No nutritional support used regardless of feeding tube placement | 63 (60.0) | 47 (74.6) | 16 (38.1) | 0.0004 |
Although both esophageal and gastric malignancies are classified as being upper gastrointestinal, nutritional recovery after resection of each is significantly different. The surgeon must consider not just the patient’s pre-operative nutritional status but the planned resection, the potential complications, and the various methods of nutritional support available. This study illustrates those tenets, with variable reliance on enteral supplementation between transhiatal and Ivor-Lewis esophagectomy and between subtotal and total gastrectomy, as well as a substantial feeding-tube related major complication rate. Older literature has suggested that feeding jejunostomy placement is a well-tolerated, low-risk additional procedure that secures enteral access following esophagectomy and total gastrectomy[9].
The operative approach to esophagectomy has its attendant risks and complication profiles. The transhiatal esophagectomy is thought to accept a higher rate of lower-grade morbidity in that a cervical anastomosis is more likely to leak but is less detrimental to the patient. Meanwhile, the Ivor-Lewis approach is believed to provide a lower likelihood of anastomotic leak with the understanding that such a leak is more devastating given the resultant mediastinitis. Of note, randomized controlled trials have not borne out such beliefs[17]. In our series, the Ivor-Lewis approach to esophagectomy was associated with lower feeding tube utilization rates at discharge compared to the transhiatal approach (25.0% vs 59.8%, respectively; P < 0.0001). As the inability to use the reconstructed conduit is the most likely reason for need for nutritional support following esophagectomy, the difference in tube utilization rates was most likely related to lower leak rates of intrathoracic anastomoses (7.1%) vs cervical anastomoses (31.7%).
The extent of gastric resection determines the reconstruction approach, typically either Billroth II gastrojejunostomy following subtotal gastrectomy or Roux-en-Y esophagojejunostomy following total gastrectomy. The lack of a gastric remnant eliminates the accommodating reservoir function of the stomach and requires a second anastomosis involving the small bowel. For these and other reasons, feeding jejunostomy placement is often routinely performed in conjunction with total gastrectomy and more selectively done with subtotal gastrectomy. In our series, feeding jejunostomy tube placement was more frequently placed during total than subtotal gastrectomy (92.9% vs 12.7%, P < 0.0001). Despite the significant difference in the frequency of feeding tube placement, tube utilization rates at the time of discharge were similar (51.3% vs 62.5%, respectively; P = 0.56). While the majority of patients who undergo subtotal gastrectomy will recover without requiring nutritional support, the relatively high tube utilization rate likely reflects a preference for enteral nutritional support instead of parenteral support when enteral access has already been established. This is evidenced in that no patient who underwent subtotal gastrectomy with feeding tube placement also received parenteral nutrition.
Our traditional institutional practice has been to routinely place FJT at the time of esophagectomy, while tube placement at the time of gastric resection has been more selective, with a higher rate of feeding jejunostomy following total gastrectomy than subtotal resection. Intra-operative feeding jejunostomy placement does not guarantee consistent enteral access or obviate the need for parenteral nutrition for postoperative supplementation. In the esophagectomy group, seven patients (3.9%) received parenteral nutrition to meet caloric goals since four patients developed chylothorax and three patients had their feeding jejunostomy removed at reoperation for intra-abdominal complications. Following gastrectomy, eleven of forty-seven patients (23.4%) who underwent feeding tube placement required parenteral nutrition. Six of these patients were given parenteral nutrition as a direct result of having developed tube-related major complications requiring reoperation. Of the remaining five patients, three had other intra-abdominal complications precluding tube feed administration and two demonstrated tube feed intolerance. Meanwhile, eleven of fifty-eight patients (19.0%) who underwent gastric resection without feeding jejunostomy placement required parenteral nutrition as a bridge to adequate oral caloric intake.
Feeding tube-specific complication rates within 30 d were identified in seven of 228 patients (3.1%), which is consistent with rates published in other series. However, nearly all tube-related complications occurred following gastrectomy, for a complication rate of 12.8% (6 of 47), all of whom had undergone total gastrectomy. All tube-related complications were major, requiring invasive procedure or reoperation for indications such as bowel ischemia, bowel perforation, or acute obstruction. This tube complication rate might be considered higher than expected, but it is consistent with the study by Llaguna et al[18] in which 18 of 73 patients (24.7%) experienced a jejunostomy tube-related complication, with 10 patients (13.7%) experiencing a complication requiring reoperation or interventional radiology procedure. In addition, Patel et al[19] demonstrated that in a population of 132 patients who underwent total or subtotal gastrectomy, feeding jejunostomy placement was associated with a greater frequency of any grade complication (59% vs 41%, P = 0.04) and specifically any infectious complication (36% vs 17%, P = 0.01). Of note, the rate of major complications was not significantly different, and the authors did not separately identify tube-related complications. Only tube placement was associated with post-operative complications on multivariate analysis, whereas age, functional status, T stage, N stage, and extent of resection were not. The higher rate of tube-specific complications following total gastrectomy compared to subtotal gastrectomy or esophagectomy in the absence of technical error suggests an inherent difference in post-operative recovery. The combination of the lack of a gastric remnant with the performance of D2 lymphadenectomy and Roux-en-Y reconstruction may place the small bowel at greater risk of impaired recovery and therefore greater likelihood of tube-related complications.
Overall tube utilization rates at discharge were on the order of fifty percent for both esophageal and gastric resection. While the optimal time for placing a feeding jejunostomy tube is at the time of resection, this does not mean that it should be done solely for sake of ease or potential prophylaxis, as half of patients will recover to discharge without the need for prolonged tube feeds. Specific resections were associated with need for tube feed supplementation, as patients who underwent transhiatal esophagectomy more frequently required nutritional supplementation at that time of discharge compared to Ivor-Lewis esophagectomy (59.8% vs 25.0%, respectively; P < 0.0001). A similar distinction was also seen when comparing total and subtotal gastrectomy patients (61.9% vs 25.4% respectively, P = 0.0004).
Parenteral nutrition has its own risks, such as central line sepsis, but has an advantage in that the decision to administer nutritional support may be postponed until the postoperative phase of recovery, when patients’ early postoperative courses can better indicate a need for such support. An alternative method of enteral access that is receiving more attention is nasojejunal tube placement at operation[13,14]. This modality is less invasive than jejunostomy tubes or central lines with fewer associated complications, but is more aimed towards supplemental nutrition while the patient is in-house as opposed to long-term. Since the placement of a nasojejunal tube adds essentially no morbidity to the operation, our practice has shifted to routinely place these tubes at the time of total or subtotal gastrectomy in order to provide nutritional support.
Given suboptimal tube utilization rates, significant feeding tube-related complication rates, and the presence of alternative methods of nutritional supplementation, we would argue that feeding jejunostomy placement should not be considered an obligatory component of any upper gastrointestinal resection. Although this study is prospective in nature, it is limited in its generalizability to patients with upper gastrointestinal malignancy. Despite that, our data suggest that the majority of patients who undergo Ivor-Lewis esophagectomy or subtotal gastrectomy will recover adequate oral caloric intake in the short term. In addition, enteral supplementation via nasojejunal tube placement may be a preferable method of nutritional delivery following total gastrectomy. By reducing the frequency of feeding jejunostomy placement, tube-related complications would be minimized and tube utilization rates would be improved. How best to predict the need and optimal route for post-operative nutritional support would be optimally assessed in a randomized, prospective manner.
In conclusion, nutritional recovery following upper gastrointestinal resection for malignancy must be assessed according to the specific pathology being treated. Esophagectomy and gastrectomy have different risks based on operative approach and complication profiles. Feeding jejunostomy was associated with significant tube-related complications, particularly following total gastrectomy. This study suggests that jejunostomy tube placement is not obligatory following upper gastrointestinal resection for malignancy and that alternative methods of nutritional supplementation such as parenteral nutrition or nasojejunal tube placement are potentially better tolerated and allow enhanced patient selection for nutritional support.
Adequate nutrition has been demonstrated to be critical to the recovery process after major resection. Various methods of nutritional support may be employed, including but not limited to parenteral nutrition, nasojejunal tube feeds, or jejunostomy tube feeds. At many institutions, feeding jejunostomy tubes (FJT) are often placed as a matter of routine in conjunction with resection of upper gastrointestinal malignancy in order to gain enteral access for support during the immediate post-operative phase as well as in anticipation of adjuvant chemotherapy. This study evaluated the actual utilization rates of such feeding tubes upon discharge as well as to assess tube-related complication rates.
Feeding jejunostomy has been widely studied in esophageal resection, but limited literature has evaluated them in major gastric resection. Although both esophageal and gastric malignancy are in the upper gastrointestinal tract, they are unique neoplasms and comparing utilization rates in each patient population has not been done to date.
In this study, tube utilization rates at discharge for both patient populations were on the order of 50%. However, utilization rates were higher in the subpopulations of total gastrectomy and transhiatal esophagectomy. Major tube-related complications were 3.1%; these were predominantly experienced by patients who underwent total gastrectomy. Meanwhile, Ivor-Lewis esophagectomy and subtotal gastrectomy patients were more likely to achieve adequate oral nutritional intake prior to discharge home.
This study suggests that nasojejunal feeding tube placement may be a preferred route of nutritional support over feeding jejunostomy following Ivor-Lewis esophagectomy and subtotal gastrectomy. This method of nutritional delivery has potential benefit as well for transhiatal esophagectomy and total gastrectomy patients, while avoiding the complications related to feeding jejunostomy placement, with consideration of parenteral nutrition as an alternative route if nasojejunal tube feeds are not able to be administered.
The authors of this paper evaluated feeding jejunostomy utilization for esophagectomy and gastrectomy for malignancy. Suboptimal utilization rates and significant tube-related major complications suggest that alternative methods of nutritional support to routine feeding jejunostomy placement allow enhanced patient selection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ker CG, Petronella P, Rausei S S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 2. | van der Schaaf MK, Tilanus HW, van Lanschot JJ, Johar AM, Lagergren P, Lagergren J, Wijnhoven BP. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg. 2014;147:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr. 2007;31:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Anastasilakis CD, Ioannidis O, Gkiomisi AI, Botsios D. Artificial nutrition and intestinal mucosal barrier functionality. Digestion. 2013;88:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Martins JR, Shiroma GM, Horie LM, Logullo L, Silva Mde L, Waitzberg DL. Factors leading to discrepancies between prescription and intake of enteral nutrition therapy in hospitalized patients. Nutrition. 2012;28:864-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | De Jonghe B, Appere-De-Vechi C, Fournier M, Tran B, Merrer J, Melchior JC, Outin H. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered? Crit Care Med. 2001;29:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534-542. [PubMed] |
| 8. | Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358:1487-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 301] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Gerndt SJ, Orringer MB. Tube jejunostomy as an adjunct to esophagectomy. Surgery. 1994;115:164-169. [PubMed] |
| 10. | Gupta V. Benefits versus risks: a prospective audit. Feeding jejunostomy during esophagectomy. World J Surg. 2009;33:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Sica GS, Sujendran V, Wheeler J, Soin B, Maynard N. Needle catheter jejunostomy at esophagectomy for cancer. J Surg Oncol. 2005;91:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ryan AM, Rowley SP, Healy LA, Flood PM, Ravi N, Reynolds JV. Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin Nutr. 2006;25:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Torres Júnior LG, de Vasconcellos Santos FA, Correia MI. Randomized clinical trial: nasoenteric tube or jejunostomy as a route for nutrition after major upper gastrointestinal operations. World J Surg. 2014;38:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Elshaer M, Gravante G, White J, Livingstone J, Riaz A, Al-Bahrani A. Routes of early enteral nutrition following oesophagectomy. Ann R Coll Surg Engl. 2016;98:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines for Nutrition Support in Cancer Patients: nutrition screening and assessment. Nutr Clin Pract. 2008;23:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Martin RC, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002;236:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Kayani B, Jarral OA, Athanasiou T, Zacharakis E. Should oesophagectomy be performed with cervical or intrathoracic anastomosis? Interact Cardiovasc Thorac Surg. 2012;14:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Llaguna OH, Kim HJ, Deal AM, Calvo BF, Stitzenberg KB, Meyers MO. Utilization and morbidity associated with placement of a feeding jejunostomy at the time of gastroesophageal resection. J Gastrointest Surg. 2011;15:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Patel SH, Kooby DA, Staley CA, Maithel SK. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol. 2013;107:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |