Published online Jul 27, 2016. doi: 10.4240/wjgs.v8.i7.521
Peer-review started: February 14, 2016
First decision: March 21, 2016
Revised: April 5, 2016
Accepted: April 21, 2016
Article in press: April 22, 2016
Published online: July 27, 2016
Processing time: 151 Days and 18.7 Hours
AIM: To evaluate published trials examining oral post-operative protein supplementation in patients having undergone gastrointestinal surgery and assessment of reported results.
METHODS: Database searches (MEDLINE, BIOSIS, EMBASE, Cochrane Trials, Cinahl, and CAB), searches of reference lists of relevant papers, and expert referral were used to identify prospective randomized controlled clinical trials. The following terms were used to locate articles: “oral’’ or “enteral’’ and “postoperative care’’ or “post-surgical’’ and “proteins’’ or “milk proteins’’ or “dietary proteins’’ or “dietary supplements’’ or “nutritional supplements’’. In databases that allowed added limitations, results were limited to clinical trials that studied humans, and publications between 1990 and 2014. Quality of collated studies was evaluated using a qualitative assessment tool and the collective results interpreted.
RESULTS: Searches identified 629 papers of which, following review, 7 were deemed eligible for qualitative evaluation. Protein supplementation does not appear to affect mortality but does reduce weight loss, and improve nutritional status. Reduction in grip strength deterioration was observed in a majority of studies, and approximately half of the studies described reduced complication rates. No changes in duration of hospital stay or plasma protein levels were reported. There is evidence to suggest that protein supplementation should be routinely provided post-operatively to this population. However, despite comprehensive searches, clinical trials that varied only the amount of protein provided via oral nutritional supplements (discrete from other nutritional components) were not found. At present, there is some evidence to support routinely prescribed oral nutritional supplements that contain protein for gastrointestinal surgery patients in the immediate post-operative stage.
CONCLUSION: The optimal level of protein supplementation required to maximise recovery in gastrointestinal surgery patients is effectively unknown, and may warrant further study.
Core tip: Malnutrition in hospitalized patients can negatively impact recovery; protein and energy deficiencies have been documented in gastrointestinal surgery patients and trials have demonstrated benefits of perioperative nutritional strategies, although post-operative oral nutritional supplementation have been studied to a lesser extent. The outcome of our work is that clinical trials that varied only the protein provided via oral supplements were not found. There is evidence to support oral protein supplements for gastrointestinal surgery patients immediately post-operatively. But the optimal level of protein supplementation required to maximise recovery in gastrointestinal surgery patients is effectively unknown, and may warrant further study.
- Citation: Crickmer M, Dunne CP, O’Regan A, Coffey JC, Dunne SS. Benefits of post-operative oral protein supplementation in gastrointestinal surgery patients: A systematic review of clinical trials. World J Gastrointest Surg 2016; 8(7): 521-532
- URL: https://www.wjgnet.com/1948-9366/full/v8/i7/521.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i7.521
Malnutrition has been associated with increased incidence of complications such as sepsis, pneumonia, wound infections, clotting disorders, and wound dehiscence[1,2]. Patients undergoing major gastrointestinal surgery can suffer periods of undernourishment, not only as a consequence of their presenting illness, but also due to reduced food intake and the resulting catabolic state that prevails in the post-operative period. In this population, malnutrition has been found to increase post-operative morbidity and mortality rates as well as the duration and subsequent cost of hospital stay[3,4]. These despite multimodal nutritional regimens, include pre-operative carbohydrate loading.
In this context, nutritional supplementation has been suggested as a routine post-operative procedure for gastrointestinal surgery patients given the putative negative nitrogen balance. Indeed, intervention in the form of post-operative protein supplementation (in the context of allowable free-fluids or light diet by mouth) has remained of interest as an effective way to improve patient recovery despite an apparent paucity of trials adequately addressing the optimum, or even appropriate, quantity of proteins or peptides. As the physiological nitrogen balance is affected by both energy and protein consumption, knowledge of the levels of each that best enable avoidance of catabolic loss after gastrointestinal surgery could benefit patient outcomes[5].
Therefore, in this review, clinical trials of oral nutritional supplements providing increased protein levels relative to controls, administered to human patients recovering from gastrointestinal surgery, were systematically assessed with respect to clinical efficacy and cost-effectiveness.
The following terms were used to locate articles: “oral’’ or “enteral’’ and “postoperative care’’ or “post-surgical’’ and “proteins’’ or “milk proteins’’ or “dietary proteins’’ or “dietary supplements’’ or “nutritional supplements’’. In databases that allowed added limitations, results were limited to clinical trials that studied humans, and publications between 1990 and 2014. Despite these limits, multiple non-clinical trial results, non-human trials, and irrelevant studies appeared and were excluded. The search was intentionally broad as more specific searches for gastrointestinal surgery associated keywords and MeSH terms resulted in numerous missed relevant papers. Databases searched included: MEDLINE, Biosis, EMBASE, Cochrane Trials, Cinahl, and CAB. Other means of identifying records included searching reference lists of relevant papers.
Randomized controlled clinical trials examining protein-based oral dietary supplementation post-operatively in human gastrointestinal surgery patients were selected. Studies were excluded if: Involved immunonutrition; related to supplementation with incomplete proteins; did not specify the amount of protein supplemented; supplemented patients pre-operatively only; not strictly oral nutrition; or not published in English.
Primary outcomes included the effect of supplementation on post-operative complications, length of hospital stay, nutritional status, and weight loss. Secondary outcomes included the effect on plasma proteins, quality of life, function, and cost of care.
Trials that met the inclusion criteria were independently assessed for eligibility by the authors (Crickmer M, O’Regan A, Dunne CP) and discrepancies were resolved by discussion. Papers were read independently by the authors and themes were identified. Risk of bias was assessed by determining allocation concealment for participants, the staff and assessors. The effect of treatment was assessed relative to clinical importance and statistical significance, using P values of ≤ 0.05 as the cut-off point. The evaluation of the trials was guided thematically with the qualitative tool, modified from a previous Cochrane Systematic Review[6], described in Tables 1 and 2. The characteristics of each study were tabulated and are shown in Table 3.
| Items and scores |
| Was the assigned treatment adequately concealed prior to allocation? |
| 2 = method did not allow disclosure of assignment |
| 1 = small but possible chance of disclosure of assignment or states random but no description |
| 0 = quasi-randomized |
| Were the outcomes of participants who withdrew described and included in the analysis (intention to treat)? |
| 2 = intention-to-treat analysis based on all cases randomized possible or carried out |
| 1 = states number and reasons for withdrawal but intention-to-treat analysis not possible |
| 0 = not mentioned or not possible |
| Were the outcome assessors blinded to treatment status? |
| 2 = action taken to blind assessors, or outcomes such that bias is unlikely |
| 1 = small or moderate chance of unblinding of assessors |
| 0 = not mentioned |
| Were the treatment and control group comparable at entry? |
| 2 = good comparability of groups |
| 1 = confounding small |
| 0 = large potential for confounding, or not discussed |
| Were care programs, other than the trial options, identical? |
| 2 = care programs clearly identical |
| 1 = clear but unimportant differences |
| 0 = not mentioned or clear and important differences in care programs |
| Were the inclusion and exclusion criteria clearly defined? |
| 2 = clearly defined |
| 1 = inadequately defined |
| 0 = not defined |
| Were the interventions clearly defined (including estimates of nutritional value)? |
| 2 = clearly defined interventions are applied with a standardized protocol |
| 1 = clearly defined interventions are applied but the application protocol is not standardized |
| 0 = intervention and/or application protocol are poorly or not defined |
| Were the participants blind to assignment status following allocation? |
| 2 = effective action taken to blind participants |
| 1 = small or moderate chance of unblinding participants |
| 0 = not possible, or not mentioned (unless double-blind), or possible but not done |
| Were the treatment providers blind to assignment status? |
| 2 = effective action taken to blind treatment providers |
| 1 = small or moderate chance of unblinding of treatment providers |
| 0 = not possible, or not mentioned (unless double-blind), or possible but not done |
| Was the overall duration of surveillance clinically appropriate? |
| 2 = optimal (six months or more) |
| 1 = adequate (one up to six months) |
| 0 = not defined, or not adequate |
| Smedley et al[8] | Saluja et al[10] | Beattie et al[13] | MacFie et al[9] | Jensen et al[5] | Keele et al[11] | Rana et al[12] | |
| Was the treatment adequately concealed prior to allocation? | 2 | 0 | 2 | 0 | 0 | 0 | 2 |
| Were candidates who withdrew included in analysis? | 0 | No withdrawals | 0 | 0 | 0 | 0 | 1 |
| Were the assessors blinded? | 0 | 0 | 0 | 0 | 2 | 0 | |
| Were treatment and control groups comparable at entry? | 2 | 2 | 0 | 2 | 1 | 2 | 2 |
| Specific mention of “lack of detail on patients at entry” | Specific mention of error “in the randomisation process” | ||||||
| Were care programmes otherwise identical? | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Were the inclusion and exclusion criteria clearly defined? | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Were the interventions clearly defined? | 2 | 2 | 2 | 1 | 1 | 2 | 2 |
| Were the participants blind to assignment after allocation? | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Were the treatment providers blinded to allocation status? | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Was duration of surveillance appropriate? | 1 | 1 | 2 | 2 | 1 | 1 | 0 |
| Ref. | Method | Participants | Intervention | Outcomes | Conclusions/Notes |
| Smedley et al[8] | RCT | Undergoing elective moderate-major lower GI surgery n = 89; 39 patients were controls in the pre-op phase and in the TG in phase 2 making them the treatment group for this analysis. Fifty patients were controls throughout making them the control group | Included multiple groups with patients receiving pre and post-operative supplementation. This review focused on the group that received no supplements pre- and post-op, as well as the group that received no supplement pre-op and supplements post-operation (CG = Group 4, TG = Group 3) | Complications: Fewer minor complications in TG (P < 0.05) | The patients in this study had a baseline of good nutritional intake |
| Supplementation began when patients allowed light diet or free fluids post-operation. | Length of stay: No difference | ||||
| Fortisip, nutricia used as supplement: 0.05 g/mL protein, 1.5 kcal/mL energy | Weight loss: Significant reduction in patients given ONS before and after surgery and in patients given postoperative ONS only | ||||
| TG asked to drink as they desired in addition to meals | Quality of life: No difference (Short Form 36, EuroQol instruments were used) | ||||
| Cost: Reduced by GBP£300 (15%) per patient, however not statistically significant | |||||
| Post-surgery oral nutritional supplements were of benefit independently of nutritional status | |||||
| Saluja et al[10] | PRCT | n = 60 (30/30) divided into BM, MM and SM using the NRI[26] | 0.033 g/mL of protein or 16.66 g/500 mL drink and 500 kcal energy, in addition to ward diet. Ward diet only was provided to control group. Trial started once surgical team allowed fluids or light diet | Adverse events: ONS well tolerated | Severely malnourished patients have increased energy requirements and less oral intake, and will therefore lose lean body mass as a substrate for energy |
| Age: Between 20-60 yr | Total protein intake: Increased in TG (P < 0.01) | Albumin half-life is 20 d - early post-op period is too short to demonstrate a difference due to supplementation | |||
| Elective and emergency abdominal procedures (not just GI) | Voluntary protein intake higher though not significant | ||||
| Treatment started from day-1 post-operatively | Weight loss: TG = 2.15 kg vs CG = 4.6 kg (P < 0.01) | ||||
| Assessment was done on admission, day 3 and at discharge | Overall weight loss: TG = 5.6%, CG = 6.4% | ||||
| Severely Malnourished Patients: TG = 6.3%, CG = 10% (P < 0.01) | |||||
| No significant change in lymphocyte count | |||||
| Complications: No significant difference | |||||
| Length of stay: Statistically significant reduction in severely malnourished patients. No difference in other categories in length of stay | |||||
| No change in mid arm circumference | |||||
| No change in hand grip strength | |||||
| Treatment group felt better than control group (subjective assessment) | |||||
| No difference in voluntary intake in group consuming supplements | |||||
| Beattie et al[13] | PRCT | Patients had a BMI < 20 or > 5% weight loss between hospital admission and trial inclusion, and other anthropometric criteria. | Oral nutritional supplement containing 0.06 g/mL protein, 1.5 kcal/mL energy. Patients encouraged to consume 400 mL/d postoperatively. In practice, patients had between 200 and 400 mL/d in addition to normal meals | Weight loss: CG lost an average maximum of 5.96 kg at 8 wk after admission, while TG lost a maximum of 3.4 kg on average in the first 4 wk and then gained weight (P < 0.001) | Support for nutritional intervention in patients with malnutrition: |
| Age: 18-80 | Mean body weight loss = 9.8% in CG and 5.6% in treatment group | Post-operative oral nutritional supplementation improved nutritional status, quality of life and morbidity | |||
| n = 101, intervention = 52; control = 49 | Triceps skin fold and MAMC were higher in TG than CG (P < 0.001) | ||||
| Function: Improved grip strength at 10 wk (P < 0.001) | |||||
| Quality of life (UK SF-36): Statistically significant improvement in mental and physical health (P < 0.001) | |||||
| Complications: Reduced (P < 0.05) | |||||
| No difference in infection rates | |||||
| Length of stay: No difference | |||||
| MacFie et al[9] | PRCT | Major GI surgery patients n = 52; 27 had intervention of some kind. TG n = 27 (post op supplements), CG n = 25 | Pre and post-operative phases. For this review, only the control group and post-operative supplementation group were looked at | Nutritional intake: Increased protein and energy seen (but no benefits could be seen) | Similar intakes of supplements as previous trials (Rana, Keele) that showed benefit |
| Fortisip, Nutricia given - 0.05 g/mL protein and 1.5 kcal energy/mL or an alternative Fortijuice, Nutricia containing 0.025 g/mL protein and 1.25 kcal/mL energy. Patients encouraged to consume 400 mL/d in addition to normal ward diet | Morbidity: No difference | Also concluded: No difference in benefit when looked at the 17 malnourished patients in the study | |||
| Supplementation commenced as soon as permitted fluids post-surgery, usually within 24 h | Mortality: No difference | Possible lack of difference due to small study numbers in each group, or in general, early return to eating post-surgery in practice along with dietician support normally at the hospital | |||
| CG was provided standard ward diet | Effect on voluntary food intake: No difference | ||||
| Nutritional status: No difference | |||||
| Functional status: No difference | |||||
| Hospital stay: No difference | |||||
| Weight Loss: No significant difference | |||||
| Serum albumin: No significant difference | |||||
| Psychological Status: No significant differences | |||||
| Return to normal activities at 6 mo: No difference | |||||
| No evidence that increased supplements decreased amount of ward diet eaten | |||||
| Jensen and Hessov[5] | RCT - Supplements given after discharge from colorectal surgery for 4 mo | Elective and acute n = 87: 47 in CG and 40 in TG | Control group: Discharged without advice | Body mass: (50 d after discharge) lean body mass increase seen in TG of 1.3 kg (P = 0.009) and in overall body mass 2.0 kg (P = 0.005). 110 d after discharge: Total mass difference was +2.7 kg for TG relative to CG (P = 0.014), and lean body mass +1.4 kg for TG (P = 0.029) No significant difference in fat mass was seen at either stage | Initially patients in the intervention group gained LBM without fat mass; later there were gains in both types of mass |
| TG: Dietetic advice and a variety of supplements including protein only - aiming for 1.5 g protein/kg per day | Serum albumin: No difference was seen at any time | Recommendation: Patients should increase protein intake to 1.5 g/kg per day for 2 mo post-surgery | |||
| Keele et al[11] | Short and long term (4 mo after discharge) benefits of intervention | n = 100 moderate-major elective GI surgery; n = 53 in CG and n = 47 in TG. | TG was given oral nutritional supplement post-operatively in addition to ward diet, which was given to the control group | Inpatient phase | Phase 1 assessment was at day 3 and discharge |
| In-patient and out-patient phases (to 4 mo after discharge) | Nutrient intake: Significant increase in protein and energy intake increase at days 1 and 2 (P < 0.001) and 3 (energy P < 0.01, protein P < 0.001), day 4 (P < 0.05) and day 7 - protein only (P < 0.05) | Clinically significant benefits with short term supplementation but not long term supplementation | |||
| (There were four groups in this study: C/C had no supplementation before/after surgery; C/S had none before and supplementation after; S/C had supplementation before and none after; S/S had supplementation before and after surgery | Supplement consumption was “ad libitum” | No significant difference in intake of energy or protein from ward diet | Both CG and TG had below requirement levels of protein as in-patients | ||
| For the purposes of the review C/C were taken as CG and C/S were taken as TG) | Supplements - 200 mL cartons of Fortisip with 1.5 kcal and 0.05 g/mL (10 g protein/carton) | Energy intake 1 m after discharge: Significantly higher in TG | By 1 mo, patients in both groups were eating well so supplements had little effect on well-being | ||
| Weight loss: Less in treatment group at day 3 and discharge (P < 0.001) | The rapidity of the effects of protein supplementation suggests that its effect is due to a direct action of key nutrients rather than repletion of tissue stores | ||||
| Serious complications: Less in treatment group (P < 0.05) | |||||
| Handgrip: Significant reduction in CG at days 3 and 7; strength lost at day 3 in treatment group but regained by discharge | |||||
| Subjective fatigue: Increased fatigue in CG at day 3 and discharge (P < 0.001), no significant increase in fatigue in TG | |||||
| Complications: More in control group (P < 0.05) | |||||
| Giving food did not reduce voluntary food intake | |||||
| Outpatient phase | |||||
| Nutrient Intake: No significant difference in protein intake in the out-patient phase. Significantly higher energy intake was seen (P < 0.05) in groups consuming supplements post-discharge compared to controls | |||||
| No benefit was seen with supplementation post-discharge | |||||
| Rana et al[12] | Short term only: Started on day patients could receive free fluids until discharge | n = 40; 20 control and 20 supplemented | Ad libitum supplementation with oral nutritional sip feed in addition to control diet | Nutritional intake: significantly higher energy intake in the treatment group P < 0.004 (as well as the nutritional value of the supplements, more energy was consumed from ward diet by these patients) and protein intake (due solely to supplements) | In the CG there is a significant protein deficit by day 3 which persisted to day 7 (often the day of discharge) |
| Major G-I surgery | 7.8 g/L unhydrolysed protein. 1.5 kcal/mL energy density. 1.4 L is needed to provide all required nutrient as defined by United Kingdom health board | Significant weight loss in CG but not TG at day 3 and discharge | 5-6 d on average elapsed between day of operation and day 1 of study period where diet was allowed. Study period began when surgical team allowed “free-fluids or light diet” | ||
| Controls and given ward diet and allowed snacks | Grip strength difference at day 3 and discharge (P < 0.03) in favor of treatment group | Within 3 d of “free fluids/light diet” treatment patients were consuming 70 g protein/d and about 2000 kcal | |||
| No difference in mid-arm circumference/triceps skin folds changes between groups | Observed increased number of calories (not protein) being eaten from ward diet in the treatment group - inference that supplementation helped to maintain appetite | ||||
| Serious complications (pneumonia, wound infection) significantly higher in CG | |||||
| No difference in length of stay | |||||
| Complications: Pneumonia and wound infection seen. P < 0.02 in favor of treatment group | |||||
| Blood proteins: No difference in serum albumin, retinol binding albumin, prealbumin. Significant difference in retinol binding protein as CG declined while TG levels remained same. P < 0.05 | |||||
| Hospital stay length: No statistically significant difference |
Where studies included both pre-and post-operative supplementation interventions, only the post-operative components are considered in this review. The numbers of trial participants per study were adjusted to reflect this.
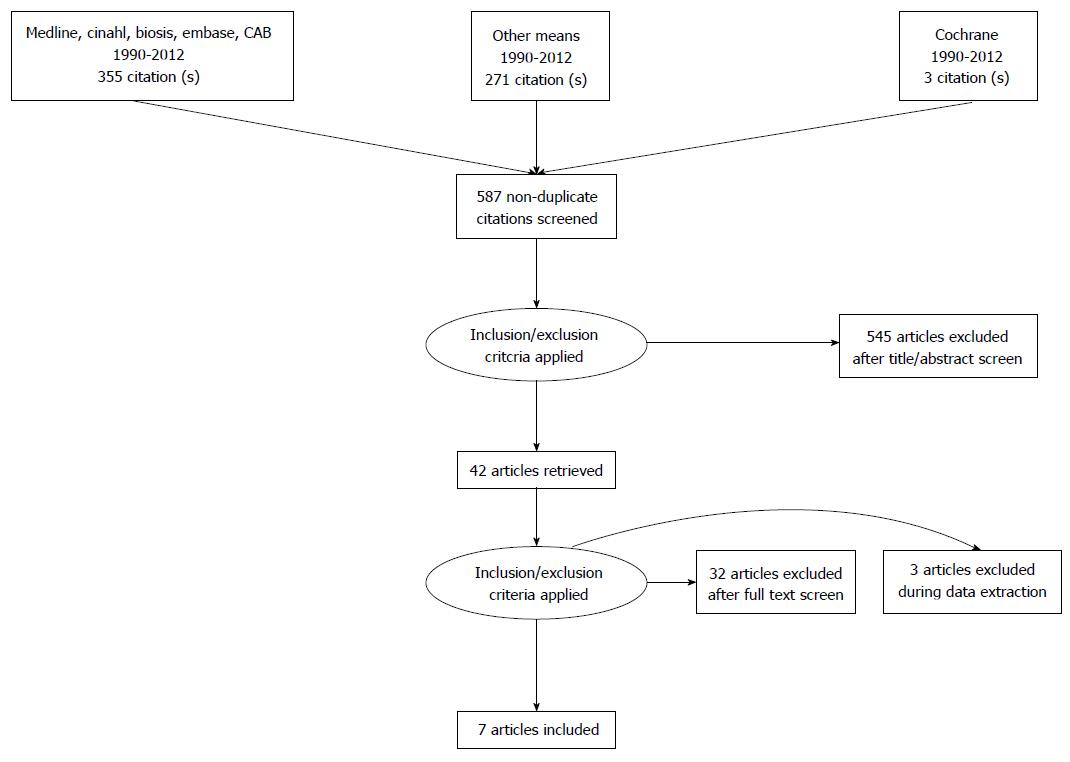
Following PRISMA guidelines[7], seven eligible reports were identified (see Figure 1). Exclusions were as follows: (1) of records found via database searching: 358; (2) of records found by other means: 271; (3) of records screened: 629; (4) of records excluded (including removal of duplicates): 587; (5) of full text articles assessed for eligibility: 42; (6) of full text articles excluded: 35; and (7) of studies included in qualitative analysis: 7.
All seven studies[5,8-13] were prospective randomized controlled trials. In most cases, the patients, their carers and the assessors were not blinded. Intention to treat was not included in most studies. The control and intervention groups had similar characteristics in each trial and in all other aspects of treatment, other than the intervention. The interventions undertaken, and inclusion and exclusion criteria, were well defined in all studies. Five of the seven studies[5,8,9,11,13] included outpatient phases that lasted between one and six months. The characteristics of the studies are outlined in Table 3.
Sample sizes ranged from 40 to 101. A total of 529 patients were involved, 262 of whom had an intervention. Participants were post-operative gastrointestinal surgery patients scheduled for acute or elective surgery. Nutritional status pre-operatively was variable, with some studies focused on malnourished patients[10,13]. Patient age ranged from 18 to greater than 75, and all studies included both genders.
All treatment group (TG) patients received post-operative nutritional supplementation in addition to their normal ward diet, while control groups (CGs) consumed only normal ward diet. In most cases, patients were encouraged to drink 200-400 mL of the supplement per day. Supplements comprised between 0.0078 g/mL and 0.06 g/mL of protein, with the most frequent value being 0.05 g/mL. They also provided between 0.6 kcal/mL and 1.5 kcal/mL of energy, with the most common inclusion being 1.5 kcal/mL. All studies, except Jensen[5], began supplementation as soon as allowed by the surgical team, typically beginning one to six days post-operatively. Some studies focused on post-operative feeding only, while studies by Smedley et al[8] and MacFie et al[9] examined both pre-operative and post-operative feeding. Only the post-operative component of those studies is considered in this review and the numbers of trial participants has been adjusted to reflect this.
Assessment was according to the following eight themes:
Overall nutritional intake: Four of the studies quantified the difference in protein intake between treatment and CGs. Specifically, Saluja et al[10] found that protein intake was significantly higher (P < 0.01) in the group given supplementation in addition to ward diet. The TG consumed an average of 55.71 ± 11.63 g of protein per day during the study period, while the CG consumed 39.48 ± 11.14 g of protein per day (P < 0.01). These increases in protein consumption were accompanied by significant increases in the amount of carbohydrate consumed (P < 0.01). Jensen et al[5] calculated that the TG consumed 22% more protein than the CG and 16% more energy. It is noteworthy that supplementation in that trial started after discharge at about day 10, and was paired with dietetic advice, while in the previous trial by Saluja et al[10], supplementation began the first day following surgery. Keele et al[11] also found that the TG showed statistically significant increases in protein and energy consumption on study days one to four. No difference in protein intake was seen in the second phase of their trial, which examined intake for 4 mo following discharge. The inpatient results (improved overall nutritional intake) found by Rana et al[12] are similar to those found by Keele et al[11].
Weight: Four of five studies that measured this factor[5,8,11,12] found reduced weight loss in normonourished patients receiving supplementation. Keele et al[11] found decreased loss both at day 3 of the trial and at discharge (P < 0.001). Rana et al[12] found that when patients started supplements as soon as they were allowed free fluids after surgery, they maintained their weight whereas by day 3, there was significant weight loss in the CG (P < 0.05). Statistically significant reduction in weight loss was also found by Smedley et al[8] (P < 0.05). In the trial by Jensen et al[5], supplementation was not started before discharge, typically around 10 d post-operatively, but at both 50 and 110 d after discharge, the intervention group had increased total and lean body mass. No other studies looked specifically at lean body mass. Conversely, however, MacFie et al[9] reported no significant difference in weight loss between control and intervention groups overall.
Studies by Saluja et al[10] and Beattie et al[13] noted reduced weight loss in the malnourished patient population investigated in their studies, but no difference was seen by MacFie et al[9] when he isolated the 17 malnourished patients from his study. More specifically, Beattie et al[13] found that controls lost an average of 5.96 kg in 8 wk, while intervention patients lost 3.4 kg on average in the first 4 wk and then gained weight (P < 0.001). Saluja et al[10] quantified the average weight loss in the TG as 2.15 kg compared to 4.6 kg in the CG (P < 0.01).
Postoperative complications: None of the studies found a difference in mortality between control and TGs. A wide variety of other complications were looked at including chest and wound infection, sepsis, cardiac arrest, pulmonary embolism, and wound dehiscence. Three of the 6 trials that investigated complications found a reduced number in the TG: Smedley et al[8] found reduced minor complications (P < 0.05) but no difference in major complications. Both Keele et al[11] and Rana et al[12] demonstrated significantly fewer serious complications in their short term studies (P < 0.05). No difference in post operative complications was found by Saluja et al[10] or MacFie et al[9]. Saluja et al[10] did find a reduction in infectious complications in severely malnourished patients, but numbers were too small for statistical significance. Similarly, Beattie et al[13] saw a reduction in complications that was not significant when adjusting for age and sex.
Length of stay: The study by Saluja et al[10] determined a difference in length of stay between treatment and CGs in severely malnourished patients, but no difference in moderately or borderline malnourished patients. Beattie’s study[13], which also looked at malnourished patients, found no difference. The three studies[8,12,13] that evaluated length of stay in normonourished patients found no difference.
Plasma proteins: Four studies investigated plasma proteins levels[5,9,10,12], with albumin the focus of most of these studies. None of these found any significant difference in serum albumin or prealbumin. Rana et al[12] found increased retinol binding protein in the TG relative to controls at day three (P < 0.05).
Cost: Smedley et al[8] found that the use of oral nutritional supplementation, irrespective of when administered, decreased cost by £300 (sterling) per patient amounting to a 15% reduction compared to patients without supplementation. This was not statistically significant. No other study considered cost of care.
Grip strength: Three of five studies that investigated grip strength found reduced loss of grip strength in patients receiving oral nutritional supplements relative to controls. Keele et al[11] found that handgrip was maintained in the intervention group and significantly reduced in controls (P < 0.01). Rana et al[12] found a significant difference at day three and again at discharge (P < 0.05). Beattie et al[13] found similar results and noted statistical significance in the differences at week 10 (P < 0.001). Saluja et al[10] and MacFie et al[9] detected no difference in hand grip strength after supplementation.
Quality of life and fatigue: Beattie et al[13] found increased quality of life in the TG using the short form 36 questionnaire (SF-36)[14] in both mental and physical health (P < 0.001). Smedley et al[8] used SF-36 and EuroQol instruments to test quality of life and found no difference. Keele et al[11] subjectively assessed fatigue and found significant increases in control patients at study day three (P < 0.001), while TG increases in fatigue were not significant.
Previous reviews have concluded that oral nutritional supplements can have positive effects in terms of recovery of nutritional status post-operatively in conditions such as fractured neck of femur, colorectal surgery, and pancreaticoduodenectomy, among others[15-17]. In these cases, the proposed benefits could be attributed to protein supplementation (i.e., the amount of orally-consumed protein that confers the greatest benefit) combined with appropriate energy intake[18]. However, what these levels are remain unclear for patients following gastrointestinal surgery. In this review, we attempted to determine this via systematic review.
Our searches did not yield a single randomized controlled trial that adequately differentiated the effect of protein supplementation from carbohydrate supplementation. Therefore, analysis of the eligible reports was problematic. Limitations included the fact that protein and energy content in TG supplements were not equivalent in most of the studies. Indeed, only the study by Saluja et al[10] described using a fixed amount of supplement for daily consumption, while the remaining studies followed an “ad-libitum” approach. Arguably, the latter approach best mirrors “real-life” clinical scenarios, however it makes discerning the true effect of protein supplementation difficult. Furthermore, the characteristics of the patient cohorts were not equivalent between studies, confounding inter-study comparisons. For example, the study by Saluja et al[10] took place in Delhi and included a greater proportion of emergency surgery patients, and patients with tuberculosis, compared with the non-emergent procedures described in the Western European reports. Moreover, inadequate follow up time with control and TGs was common across studies, with some risk of bias associated with lack of blinding of participants, carers and assessors. The power of the studies was often too small, with one author conceding notably that “numbers were too small for meaningful statistical analysis”[13] and intention to treat analysis was not used in any of the studies. Finally, the most recent of the eligible trials found in our searches was published in 2004, arguably reflecting either a shift in interest away from oral intake in favor of enteral and parenteral nutrition in this population or an emphasis placed on ordinary diet without supplement.
Despite these limitations, the authors of six of the seven studies detailed weight loss in patients receiving post-operative nutritional supplements, but to a lesser degree than the loss in control patients[5,8,10-13]. In fact, the data suggest that this effect may be most prominent in patients malnourished initially, with statistically significant reductions in weight loss observed. While one study failed to observe this effect[9], despite similar energy and protein intakes to other trials, the authors proposed that the lack of effect was due to a small sample size. Finally, with respect to weight gain post-operatively, it appears that weight gain commenced sooner and patients appeared to return to their preoperative weight more rapidly where supplementation was provided.
There is no evidence to suggest that nutritional supplementation post-operatively reduces mortality. While more deaths did occur in the CGs of the reported trials, much larger samples would be needed to approach statistical significance. The topic of avoidance of both serious and minor complications is less clear. There is some precedent to suggest that post-operative supplementation decreases complications, as the effect has been documented in patients undergoing hip surgery[15,19,20]. However, across the eligible studies here, the rate of both serious and minor complications was significantly reduced in four of the trials[8,11-13], while no statistically significant difference was observed in two[9,10]. This variation has been addressed somewhat by Beattie et al[13] when comparing his results to the study by MacFie et al[9] in making reference to discrepancies in defining complications. On a related topic, duration of hospitalization was reduced significantly in severely malnourished patients only[10]. An additional study that supplemented increased amounts of protein and medium chain carbohydrates in enteral feed in gastrointestinal patients post-operatively found a 6-d reduction on average (P < 0.05)[21]. However, that study had larger numbers than any of the studies considered in this review, with 229 total and 115 TG patients. It has also been suggested that length of stay is a relatively poor outcome to evaluate as it often depends on patient social circumstances and services available in addition to patient post-operative clinical condition[12].
With respect to malnutrition, and assessment of patient incidence, low albumin has been used as a measurable indicator, but evidence suggests that it is not an effective marker of recent nutritional intake[22]. Approximately 5% of the circulating albumin is replaced daily by the liver and, therefore, any changes in protein intake would not be evident immediately. Further, protein markers in the blood such as prealbumin, albumin and transferrin are impacted by fluid shifts and responses to injury and inflammation which complicate their use in comparisons of patients before and after major abdominal surgery, when tissue injury is present[23]. This may explain why none of the studies in our review that monitored albumin in the early post-operative period found any disparity in albumin levels despite differences in anthropometric indicators of malnutrition (e.g., triceps skin fold, mid upper arm circumference and BMI[23]). Similarly inconsistent results were found regarding quality of life.
Evaluation of quality of life and physiological function were similarly inconsistent. For example, handgrip strength is a marker of function and results were variable across the trials. However, those variances may be explained by the use of dissimilar techniques for measurement, and confounders such as pain and fatigue post-operatively. Interestingly, the TGs in the studies conducted by MacFie et al[9] and Saluja et al[10] both described increased total body mass retention relative to CGs, but without significant increases in grip strength. Jensen et al[5] found that supplementation increased lean body mass particularly, not simply fat mass, so one could hypothesize that a functional measurement like grip strength would also be improved, but this was not described. Previous work has documented a link between impaired muscle function and nutritionally-related complications[24,25], but this was not consistently reflected across the eligible trials here.
The seven studies reviewed in this paper concurred that there is no difference in mortality seen with protein-inclusive nutritional supplements. There is evidence to suggest that weight reduction, nutritional intake, and nutritional status are improved, and that there may be positive cost of hospitalisation benefits, but evidence as to the effect on complications and grip strength is mixed. At present, there is some evidence to support routinely prescribed oral nutritional supplements that contain protein for gastrointestinal surgery patients in the immediate post-operative stage. However, randomized control trials using well-designed methodology to examine the optimal protein content needed to confer benefit are needed.
Malnutrition in hospitalized patients can negatively impact recovery; protein and energy deficiencies have been documented in gastrointestinal surgery patients and trials have demonstrated benefits of perioperative nutritional strategies, although post-operative oral nutritional supplementation have been studied to a lesser extent. The proposed benefits may be attributed to protein supplementation (i.e., the amount of orally-consumed protein that confers the greatest benefit) combined with appropriate energy intake. However, what those levels may be remain unclear for gastrointestinal surgery patients.
The positive impact of pre-operative nutrition has been reviewed previously and meta-analyses have demonstrated positive influence on patient outcome following gastrointestinal surgery. In the postoperative period however, the most pertinent question may be whether patients should be further supplemented. The most recent of the eligible trials found in the searches was published in 2004.
The authors’ searches did not yield a single randomized controlled trial that adequately differentiated the effect of protein supplementation from carbohydrate supplementation. With only one exception, the eligible studies reviewed here involved post-operative supplements administered “ad-libitum”. Although this may best mirror “real-life” clinical scenarios, it makes discerning the true effect of protein supplementation difficult. Furthermore, the characteristics of the patient cohorts were not equivalent between studies. Despite these limitations, the authors of six of the seven studies detailed weight loss in patients receiving post-operative nutritional supplements, but to a lesser degree than the loss in control patients. There is no evidence to suggest that nutritional supplementation post-operatively reduces mortality and evaluation of quality of life and physiological function were similarly inconsistent.
An intervention based on oral protein supplementation should be investigated through a double-blind randomized controlled trial.
There are no terms used that are uncommon and that would be unfamiliar to readers.
The authors have made a good collation of the data to assess if there are any benefits of post-operative protein nutritional supplementation following gastrointestinal surgery. The manuscript is well written and covers the salient points from the published studies.
P- Reviewer: Kate V, Klek S, Luyer MDP S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Burden S, Todd C, Hill J, Lal S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. 2012;11:CD008879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Hulsewe K, Von Meyenfeldt M, Soeters P, Payne-James J, Grimble G. Silk, DBA Nutrition support for the surgical patient. In: Payne-James J, Grimble G, Silk DBA, editors. Artificial nutrition support in clinical practice. 2ed. Cambridge University Press, 2001. . |
| 3. | Dempsey DT, Mullen JL, Buzby GP. The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J Clin Nutr. 1988;47:352-356. [PubMed] |
| 4. | Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996;12:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 258] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Jensen MB, Hessov I. Dietary supplementation at home improves the regain of lean body mass after surgery. Nutrition. 1997;13:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Parker MJ, Gurusamy KS, Azegami S. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev. 2010;CD001706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Swartz MK. The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care. 2011;25:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Smedley F, Bowling T, James M, Stokes E, Goodger C, O’Connor O, Oldale C, Jones P, Silk D. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br J Surg. 2004;91:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | MacFie J, Woodcock NP, Palmer MD, Walker A, Townsend S, Mitchell CJ. Oral dietary supplements in pre- and postoperative surgical patients: a prospective and randomized clinical trial. Nutrition. 2000;16:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Saluja SS, Kaur N, Shrivastava UK. Enteral nutrition in surgical patients. Surg Today. 2002;32:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Keele AM, Bray MJ, Emery PW, Duncan HD, Silk DB. Two phase randomised controlled clinical trial of postoperative oral dietary supplements in surgical patients. Gut. 1997;40:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Rana SK, Bray J, Menzies-Gow N, Jameson J, Payne James JJ, Frost P, Silk DB. Short term benefits of post-operative oral dietary supplements in surgical patients. Clin Nutr. 1992;11:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut. 2000;46:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 202] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996;27:1812-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 236] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Bastow MD, Rawlings J, Allison SP. Undernutrition, hypothermia, and injury in elderly women with fractured femur: an injury response to altered metabolism? Lancet. 1983;1:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 157] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 799] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 17. | Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN, Demartines N. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:817-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 18. | Stroud M. Protein and the critically ill; do we know what to give? Proc Nutr Soc. 2007;66:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Myint MW, Wu J, Wong E, Chan SP, To TS, Chau MW, Ting KH, Fung PM, Au KS. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: a single blind randomised controlled trial. Age Ageing. 2013;42:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Botella-Carretero JI, Iglesias B, Balsa JA, Arrieta F, Zamarrón I, Vázquez C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: a randomized clinical trial. Clin Nutr. 2010;29:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Wang X, Pan L, Zhang P, Liu X, Wu G, Wang Y, Liu Y, Li N, Li J. Enteral nutrition improves clinical outcome and shortens hospital stay after cancer surgery. J Invest Surg. 2010;23:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2010;CD001880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Jeejeebhoy KN. The Practical Nutritional Assessment. In: Buchman A, editor. Clinical Nutrition in Gastrointestinal Disease. Slack Books 2006; . |
| 25. | Fritz T, Höllwarth I, Romaschow M, Schlag P. The predictive role of bioelectrical impedance analysis (BIA) in postoperative complications of cancer patients. Eur J Surg Oncol. 1990;16:326-331. [PubMed] |
| 26. | Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 953] [Article Influence: 28.0] [Reference Citation Analysis (0)] |