Published online Jul 27, 2016. doi: 10.4240/wjgs.v8.i7.492
Peer-review started: January 30, 2016
First decision: March 9, 2016
Revised: March 23, 2016
Accepted: April 7, 2016
Article in press: April 11, 2016
Published online: July 27, 2016
Processing time: 166 Days and 0.1 Hours
AIM: To compare the outcomes of a cohort of Crohn’s disease (CD) patients undergoing early surgery (ES) to those undergoing initial medical therapy (IMT).
METHODS: We performed a review of a prospective database CD patients managed at a single tertiary institution. Inclusion criteria were all patients with ileal or ileocolonic CD between 1995-2014. Patients with incomplete data, isolated colonic or perianal CD were excluded. Primary endpoints included the need for, and time to subsequent surgery. Secondary endpoints included the number and duration of hospital admissions, and medical therapy.
RESULTS: Forty-two patients underwent ES and 115 underwent IMT. The operative intervention rate at 5 years in the ES group was 14.2% vs IMT 31.3% (HR = 0.41, 95%CI: 0.23-0.72, P = 0.041). The ES group had fewer hospital admissions per patient [median 1 vs 3 (P = 0.012)] and fewer patients required anti-TNF therapy than IMT (33.3% vs 57%, P = 0.003). A subgroup analysis of 62 IMT patients who had undergone surgery were compared to ES patients, and showed similar 5 year (from index surgery) re-operation rates 16.1% vs 14.3%. In this subset, a significant difference was still found in median number of hospital admissions favouring ES, 1 vs 2 (P = 0.002).
CONCLUSION: Our data supports other recent studies suggesting that patients with ileocolonic CD may have a more benign disease course if undergoing early surgical intervention, with fewer admissions to hospital and a trend to reduced overall operation rates.
Core tip: This study supports the growing body of evidence that asserts that selected patients with ileocolonic Crohn’s have reduced requirement for medical therapy and a trend to fewer surgical interventions. Expanding on the evidence, this study also demonstrated fewer admissions to hospital for Crohn’s disease related illness.
- Citation: An V, Cohen L, Lawrence M, Thomas M, Andrews J, Moore J. Early surgery in Crohn’s disease a benefit in selected cases. World J Gastrointest Surg 2016; 8(7): 492-500
- URL: https://www.wjgnet.com/1948-9366/full/v8/i7/492.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i7.492
Surgery is often an integral part of the management algorithm for patients with Crohn’s disease (CD), with up to 20%-40% requiring surgery within in their first year[1-4] and up to 80% at some time during their disease[5]. As a chronic condition with a relapsing remitting course, surgery does not provide cure, but is an adjunct for the management of acute complications of penetrating and stricturing disease and also after maximal medical therapy fails to control disease or presents unacceptable side effects. Advances in medical therapy have seen a reduction in long-term steroid use and longer disease remission. Despite these advances older studies suggest no reduction in the proportion of patients requiring surgery. In a retrospective series, Cosnes et al[6], reported that 35% patients required surgery at 5 years, which was the same as historical cohorts.
However, the advent of biologic agents has significantly influenced the clinical course of patients of CD and also surgical decision-making. ACCENT 1 reported higher clinical remission (OR = 2.7) in patients receiving maintenance infliximab compared with placebo[7]. Similar findings have been reported for Adalimumab with higher rates of clinical remission compared with placebo (36% vs 12%)[8]. Additionally in a report from the Nationwide Inpatient Sample in the United States, rates of surgical intervention have fallen from 17.3% in 1997 to 12.4% in 2007[9].
Contention exists in the literature regarding the optimal timing of surgery in the management algorithm of CD, particularly in patients with short segment disease where resection of all macroscopic disease is feasible. Some evidence suggests that early surgery (ES) in CD may lead to a longer time to clinical recurrence[10] and lower long-term reoperation rate (14% at 5 years) compared with later surgery (30% at 5 years)[11]. Additionally, ES cohorts are reported to have reduced requirements for steroids and immunosuppression[3,11]. This study aims to determine whether patients who have ES for ileal or ileocolonic CD run a more benign clinical course, as determined by the need for fewer operations, hospital admissions and the ongoing medical therapy required for disease control than those managed with conventional medical therapy.
This study is a cohort comparison study between patients who underwent ES compared with those that underwent initial medical therapy (IMT). We examined a consecutive series of patients with ileal and ileocolonic CD managed at a major metropolitan teaching hospital from 1995 to 2014. Data were extracted from a clinical IBD database within the IBD service at the Royal Adelaide Hospital. This database was prospectively maintained from 2007, and prior to this, data were sourced from case notes review. Additional data were collected from review of medical records and pathology records.
ES was defined as patients who have undergone upfront surgery for CD due to an acute complication and those who underwent surgery within 6 mo of their diagnosis of CD. This arbitrary time frame was chosen as within this time period there is limited scope to have established of medical therapy. Acute complications included abdominal pain with peritonism, obstruction, perforation or fistulisation. The IMT cohort included patients with a histological or clinical diagnosis of CD made after 1995 referred to our health service who have undergone at least 6 mo of medical therapy. Patients diagnosed prior to this date were excluded. Patients in this cohort who went on to require bowel resection for their disease were also identified for a subgroup analysis and considered to have deferred surgery (DS).
Data collected included patient demographics, disease phenotype according to the Montreal classification[12], medical and surgical therapy. The primary endpoint for each patient was need for subsequent surgical resection. Secondary endpoints were the number of hospitalizations and days in hospital over the duration of their disease. All inpatient care data (number of admissions and total length of stay) were captured by a statewide computer database, which records admissions to all public hospitals within the state in this period.
Inclusion criteria were patients with ileal or ileo-colonic CD, with or without perianal involvement. Patients with isolated colonic or isolated perianal CD or those with incomplete records were excluded.
Data regarding patients’ medical therapy for CD were collected, but due to the retrospective nature of the database prior to 2007, the accuracy of fine details such as time course, dose and duration of therapy could not be assured. Consequently, medical therapies received by each patient are reported as a categorical outcome, described by type of treatment (none, steroid, immunosuppressive or biologic therapy).
This project was reviewed and approved by the Royal Adelaide Hospital Human Research Ethics committee. As this was a clinical audit, individual patient consent was not necessary and not sought. Data were tabulated in a Microsoft Excel™ spreadsheet. Statistical analyses were performed utilizing SPSS™ ver. 22. Differences between groups were compared using the χ2 test for categorical data, and ordinal data were compared using the Student’s t-test or Mann-Whitney-U test for non-parametric distributions. To determine a difference in time to further surgery between groups, Kaplan-Meier analyses were performed and differences tested using the Mantel-Cox log rank test.
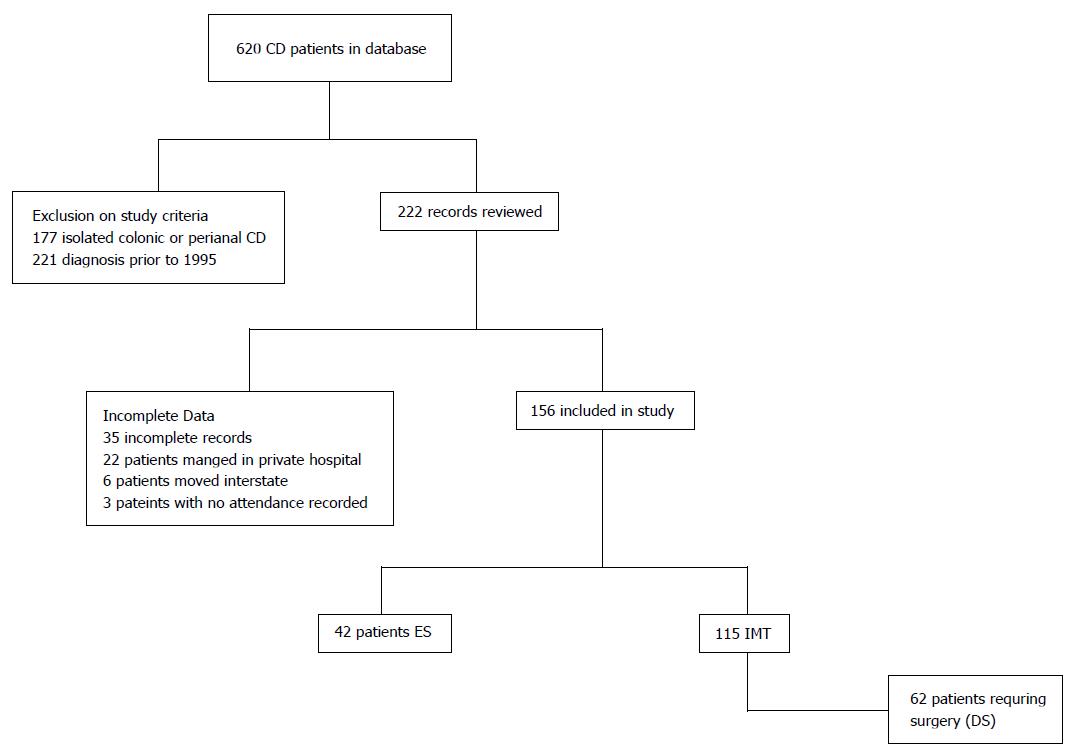
A total of 620 patients with CD were identified on the database. Exclusions are shown on a consort diagram (Figure 1). A total of 157 patients met inclusion criteria and were included in the study. There were 42 patients in the ES cohort, who either presented with an acute complication requiring emergency surgery or underwent an operation within 6 m of diagnosis due to a progression or complication of CD. The remaining 115 patients were treated with IMT, 62 (53.9%) of whom underwent surgery by the end of the study period (DS).
Demographics of the cohorts are shown in Table 1. Patients in the ES group were significantly older with a median age at diagnosis of 34.5 years (24-45) compared to 24 (19-33) in the IMT group (P = 0.0001) and a shorter duration of disease (ES 5.6 years, IMT 8.9, P = 0.014). Not surprisingly, ES patients were more likely to have a stricturing or penetrating phenotype than IMT patients. The DS cohort, who were medically treated patients who went on to have a resection, had the same proportion of patients with penetrating and stricturing disease (B2 and B3), 38.7% and 54.8% respectively as the ES patients (40.5% and 52.4% respectively P = 0.968). There was also a significant difference in disease location, with a higher proportion of L3 disease compared to L1 disease and subsequently in the proportion of patients with perianal disease in the IMT cohort.
| Initial medical therapy (n = 115) | |||||
| Clinical details | Early surgery (n = 42) | IMT (n =115) | P value | DS (n = 62)1 | P value |
| Gender M:F | 22:20 | 50:65 | 0.531 | 24:38:00 | 0.227 |
| Age at diagnosis (yr), median (IQR) | 34.5 (24-46) | 24 (19-33) | 0.0001 | 23.5 (18.25-31.75) | 0.006 |
| Smoking | 15 (35.7) | 43 (37.4) | 0.852 | 25 (40.3) | 0.685 |
| Phenotype | |||||
| B1 (non-stricturing) | 3 (7.1) | 38 (36.2) | 0.001 | 4 (6.5) | 0.968 |
| B2 (stricturing) | 17 (40.5) | 26 (24.8) | 24 (38.7) | ||
| B3 (penetrating) | 22 (52.4) | 41 (39.0) | 34 (54.8) | ||
| Location | |||||
| L1 (ileal) | 28 (66.7) | 25 (24.5) | 0.0001 | 17 (26.3) | 0.0001 |
| Perianal disease | 0 | 6 (5.2) | 3 (4.8) | ||
| L3 (ileocolonic) | |||||
| Perianal disease | 14 (33.3) | 74 (69.4) | 45 (70.2) | ||
| 5 (11.9) | 40 (34.8) | 16 (25.8) | |||
Table 2 outlines the mode of presentation of patients requiring ES. Over half the ES cohort presented with either acute obstruction or perforation necessitating emergency surgery and data was unavailable for 5 patients in this cohort. In contrast, of the patients in the DS cohort, 11 patients presented with an acute complication necessitating emergency surgery despite upfront medical therapy, 4 with acute obstruction and 7 with perforation. Thirty-five (56.5%) of the DS cohort required surgery due to progression in obstructive symptoms (6.5%) or fistula formation (24.2%).
| Clinical details | Early surgery (n = 42) | Deferred surgery (n = 62) |
| Indication for surgery | ||
| Acute obstruction – emergency resection | 9 (21.4) | 4 (23.0) |
| Subacute obstruction – elective resection | - | 20 (32.3) |
| Perforation | 15 (35.7) | 7 (11.3) |
| Fistula/phlegmon | 5 (11.9) | 15 (24.2) |
| Abdominal pain | 5 (11.9) | 2 (3.2) |
| Haemorrhage | 3 (7.1) | - |
| Not specified | 5 (11.9) | 14 (23.0) |
The number of patients in each cohort requiring surgical resection, hospital admission and medical therapy is shown in Table 3. The proportion of patients requiring subsequent resection at 5 years was significantly lower in the ES group compared with the IMT group (14.2% vs 31.3%, P = 0.041). Of note though, 57.3% of IMT patients required no surgery. Endpoints for the DS subgroup of patients were determined from the time of their index operation, allowing a fairer comparison to the ES group, as the two cohorts have all undergone one operation. The rate of subsequent surgery in the subgroup of 62 DS patients was 16.1%, which was not significantly different to the ES cohort. The median duration of between diagnosis and index operation in the DS cohort was 46.4 mo (IQR 23-97). The extent of resection and need for stoma were similar in each group and shown in Table 4.
| Initial medical therapy (n = 115) | |||||
| Clinical details | Early surgery (n = 42) | Initial medical therapy (n = 115) | P value | Deferred surgery (n = 62)1 | P value |
| Number requiring surgery | |||||
| 3 yr | 5 (11.9) | 24 (20.7) | 0.250 | 5 (8.1) | 0.521 |
| 5 yr | 6 (14.2) | 36 (31.3) | 0.041 | 10 (16.1) | NS |
| Completion of study period | 7 (16.7) | 62 (53.9) | < 0.0001 | 20 (32.3) | 0.110 |
| Number of admission to hospital per patient, median (IQR) | 1 (1-2) | 3 (1-5) | 0.012 | 2 (1-4.5) | 0.002 |
| Days in hospital (d), median (IQR) | 12.5 (9-22.5) | 11 (3-28) | 0.230 | 17 (8-28) | 0.347 |
| Medical therapy | |||||
| Immune modulator | 32 (76.2) | 101 (87.8) | 0.083 | 54 (87.1) | 0.189 |
| Steroids | 12 (28.6) | 34 (29.6) | NS | 23 (37.1) | 0.404 |
| Anti-TNF | 14 (33.3) | 69 (60.0) | 0.004 | 42 (67.7) | 0.001 |
| No requirement for medical therapy | 10 (23.8) | 5 (4.3) | 0.008 | 0 (0) | < 0.0001 |
| Follow-up months (mo), median (IQR) | 67 (31-114) | 97 (58-150) | 64 (19-121) | ||
| Clinical details | Early surgery (n = 42) | Deferred surgery (n = 62) | P value |
| Operation | |||
| Small bowel resection | 5 (11.9) | 7 (11.3) | NS |
| Ileocolic resection | 31 (73.8) | 30 (48.4) | 0.015 |
| Small bowel and segmental colonic resection | 3 (7.1) | 9 (14.5) | 0.352 |
| Small bowel and total colectomy | 2 (4.8) | 9 (14.5) | 0.193 |
| Data unavailable | 1 (2.9) | 7 (11.3) | 0.139 |
| Stoma formation | 3 (7.1) | 5 (8.1) | NS |
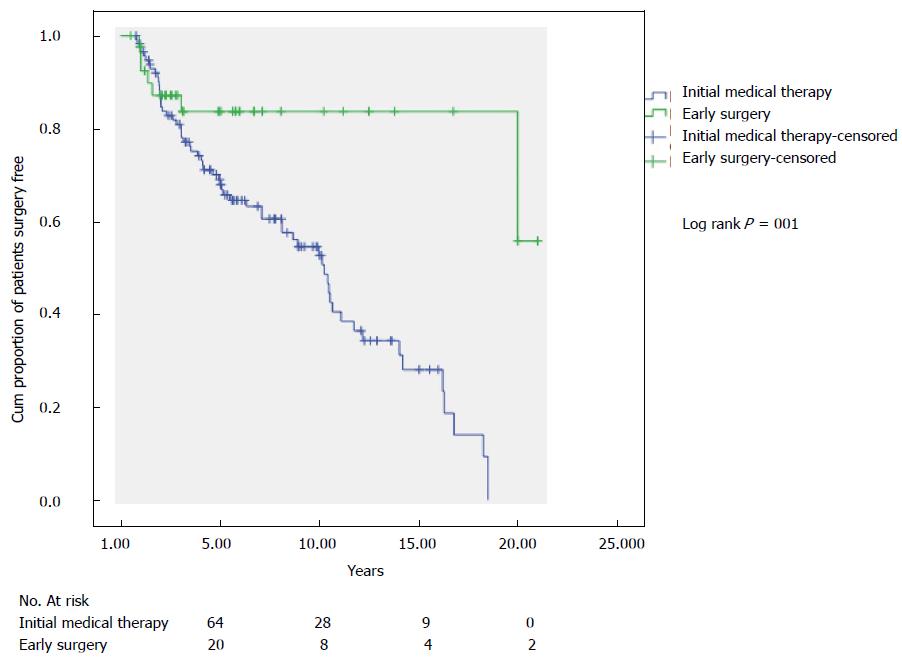
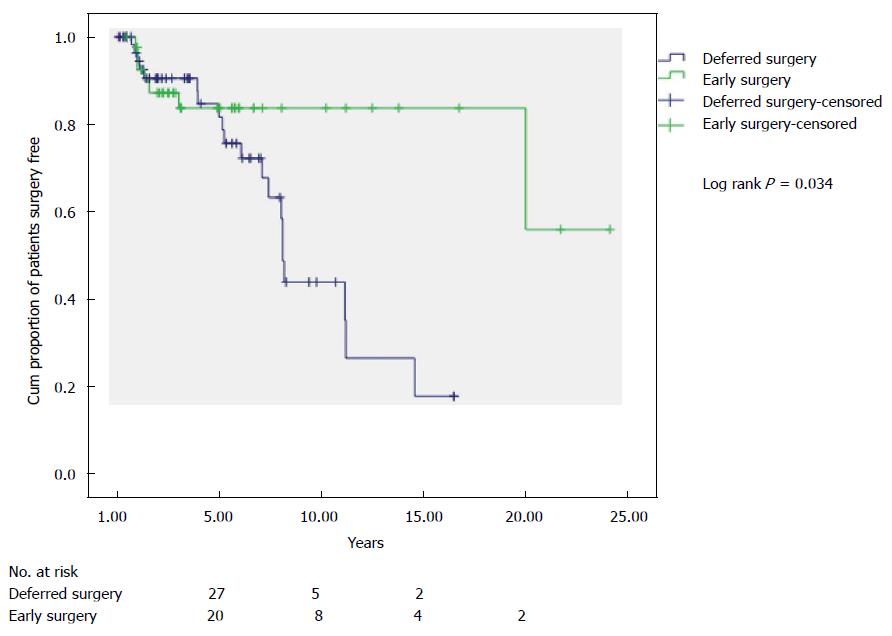
The ES group had a higher estimated proportion of patients not having a subsequent resection at 10 years than those having IMT (Figure 2) (83.7% vs 52.7%; PLog-rank = 0001). The comparison of ES to DS groups is shown in Figure 3 with an estimated probability of no subsequent surgery at 10 years of 83.7% and 43.7% (PLog-rank = 0.032) respectively.
The median number of hospital admissions differed between the two groups, with the ES group having fewer admissions than each of the IMT and DS patients (ES 1 vs IMT 3, P = 0.012) and vs (DS subset 2, P = 0.002). The median number of days in hospital over the duration of each patients’ disease did not differ significantly between the groups. However, this includes the index admission for surgery in the ES cohort.
Rates of immunomodulator and steroid use were similar when comparing the ES group to both IMT and DS cohorts. The proportion of patients receiving biologic therapy was significantly lower in the ES cohort than those having IMT (ES 33.3% vs IMT 60.0%, P = 0.004) and also the DS subgroup (ES 33.3% vs DS 67.7%, P = 0.001). The proportion of patients requiring no treatment for their disease differed between the groups (ES 23.8% vs IMT 4.3% vs DS 0%, P < 0.0001).
CD remains a challenging chronic condition where there has been a progressive evolution in the positioning and roles of medical and surgical therapy. Our study has found that 31.3% of patients initially managed with medical therapy will come to surgery within 5 years, in line with other studies[2,4,6,13]. This is significantly higher than the 5 year subsequent resection rate of those patients undergoing ES (14.2%).
An alternative interpretation of our data is that 68.7% of patients undergoing medical therapy avoided the need for surgery altogether within a 5 year period. However, the IMT cohort likely represented a less aggressive phenotype of CD as demonstrated by lower rates of stricturing and penetrating disease in this group compared with the ES group. The IMT patients who have had subsequent surgery (DS), had a similar phenotype to the ES group and comparison between these two groups may be more relevant. Indeed at 5 years, similar numbers had had further resection (ES 14.2% vs DS 16.1%), even a trend to higher rates of surgery in the DS group by the end of the study (ES 16.7% vs DS 32.3%). The estimated proportion of patients not requiring subsequent surgery at 10 years for the ES and DS groups were 83.7% and 43.7% respectively. These findings are similar to reports by Golovics et al[3] and Latella et al[11], which give validation to these results across different healthcare settings in different countries.
This is the only study to the authors’ knowledge that additionally examined inpatient healthcare utilization (hospital admissions and length total cumulative of stay) as an endpoint in comparing ES and IMT. The ES patients had fewer hospital admissions for the duration of their disease than IMT patients and the subset of DS patients [ES 67, IMT 107, DS 50 (median, months)] suggesting disease control may be better in the ES group. However a conservative interpretation of this is necessary given the longer follow-up and higher incidence of L3 and perianal disease in the IMT group.
What is difficult to quantify in the literature is the health related quality of life (HRQOL) of medically managed patients. There is a paucity of data in the literature examining this, whilst more surgical data exist with Thirlby et al[14] reporting an improvement in 7 of 8 domains in HRQOL at 12 mo after surgical resection. Casellas et al[15] reported significant differences in HRQOL scores between patients with active disease and those in disease remission. Such assessments at different time intervals of the two cohorts would be valuable in determining the true effect of ES and whether early resection conferred an improvement in quality of life and disease control.
We did not find a difference in steroid use between the groups, unlike Aratari et al[10]. who reported a need for corticosteroid therapy in 39.8% of ES patients compared with 62.1% of medically treated CD at 5 years. The similar rates of immunomodulator use we observed in ES and IMT patients, 76.2% and 87.8% respectively, are in line with current evidence supporting pro-active tailored post resection therapy to reduce clinical and endoscopic recurrence[16-18].
Immune modulators and biologic agents have added to the armamentarium of medical therapy for CD. Careful consideration should be given regarding the timing of surgery in patients with ileal CD, especially where there is a stricturing or penetrating phenotype, and this should be balanced against the aggressive pursuit of medically induced clinical remission. This balance of medical therapy and surgery may best be achieved in a multidisciplinary environment involving gastroenterologists, surgeons and radiologists. Patients with CD are now often coming to surgery on at least an immunomodulator, and in our cohort 67.7% on a biologic agent. Recent systematic reviews reported an increase in the post-operative, infective and anastomotic complications in patients on anti-TNFα agents[19,20] which lends further weight for considering earlier surgery.
It is clear that biologic agents improve rates of clinical remission and HRQOL scores in the short term[21-23]. Long term it is unclear whether they alter disease course. Disease recurrence rates at 5 years were reported at 36.6% with infliximab therapy[24] suggesting that for some the response is short lived for some patients. Resection rates in the literature are contradictory, with some studies reporting a decline in surgical resection[24,25], whilst others have reported no change despite increasing use of biologic agents[6,26-28], which may reflect the use of biologic agents at a later stage in the disease process where fibrosis and scarring predominate over inflammation, reducing their efficacy.
It is worth noting that the European evidence based consensus from the European Crohn’s and Colitis Organization recommends resection for patients with ileocolic disease with obstructive symptoms[29]. Silverstein et al[30] reported on the high costs associated with surgical intervention, accounting for 44% of the total lifetime health costs (USD17562) in a patient with CD, but also that it offered the longest remissions. However, this study pre-dates the widespread use of biologic agents and the high costs associated with them. A Canadian study estimated a direct health care cost of USD21416 per patient for the first year of Infliximab treatment[31]. No formal cost analysis was performed, however, given the lower proportion of patients requiring medical therapy in the ES cohort, the cost effectiveness of ES vs IMT should be explored as, notably 23.8% of the ES cohort avoided the need for ongoing medical therapy altogether.
There are naturally limitations to any retrospective analysis, which prevent strong conclusions being drawn. However, a study prospectively randomizing to early and DS would be difficult to conduct, require long follow up and may not be ethically acceptable. We therefore need to examine real world data such as these whilst taking account of possible sources of bias. Data was not available regarding short term complication rates of surgery and medical therapy so not included in this study. We have used the emergency operation for an acute complication as a surrogate for ES. The phenotypes of the two cohorts are different, however, we feel that the ES group had generally a more aggressive phenotype given then higher proportion of penetrating and stricturing disease presenting with an acute complication requiring resection at index presentation. The younger patients higher proportion of L3 disease in the IMT group reflects a real world cohort with potentially multifocal disease in whom the treating team have adopted medical therapy upfront. However we still believe the groups are comparable as, despite this, the type of surgery and extent of bowel resection are similar between the ES and medically treated cohorts. Our definition of ES is arbitrary, but in six months medical therapy is unlikely to be established. Being a tertiary centre, there is a potential for a referral bias, with less complex disease managed at regional centres.
Our study lends weight to the argument that in selected patients with stricturing or penetrating ileocolonic CD, those undergoing ES may have a more benign disease course, possibly with less need for further surgical intervention and fewer hospital admissions for CD related illness. This is perhaps even more meaningful, given that a significant number of these patients present with aggressive phenotypes requiring ES. Surgery should not be considered as treatment of last resort after all medical therapy has failed. Rather a more considered approach to the timing of resection for symptomatic patients is needed, possibly to achieve longer periods of disease remission with reduced drug exposure and costs to health care.
Despite the advances in medical therapy for patients Crohn’s disease (CD), from the introduction in immunomodulatory agents and biologic agents targeting TNF-α, there has been minimal decline in the rate of surgical resection in these patients. There is evidence in support of an aggressive “top-down” strategy, utilizing more effective agents earlier in the disease course such as the immunomodulators and biologic agents to control the inflammatory process and prevent progression. It must be considered whether a more aggressive approach still, with early surgery (ES) in selected patients may further improve outcomes in these patients.
The authors in selecting patients with symptomatic ileo-colonic CD, undertaking early surgical resection prior to starting medical therapy for their CD may confer a benefit in the long term, regarding the need for further surgery reduced requirement for medical therapy in maintaining control of their disease.
This study supports evidence in the literature that ES may confer a benefit in selected patients with CD, as patients required fewer admissions to hospital, spent fewer total days in hospital by the end of this study period in spite of the inclusion of their index admission for their acute presentation and operation. In addition, the authors found a longer time duration between their index operation and their subsequent operation when compared to those undergoing initial medical therapy.
Decision making in the management of patients with CD is complex and best undertaken in a multidisciplinary setting. Traditionally surgery has been reserved as an act of last resort. This study suggests a bolder approach may be of benefit in symptomatic patients with ileal or ileocolonic disease.
It is a study about an interesting topic in CD: The effectiveness of ES in avoiding CD recurrence.
P- Reviewer: Giudici F, Matsumoto S, M’Koma A, Zefelippo A S- Editor: Gong ZM L- Editor: A E- Editor: Jiao XK
| 1. | Jeon SJ, Lee KJ, Lee MH, Lim SK, Kang CJ, Kim JH. Rates of early surgery and associated risk factors in Crohn’s disease. Korean J Gastroenterol. 2010;56:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 475] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Golovics PA, Lakatos L, Nagy A, Pandur T, Szita I, Balogh M, Molnar C, Komaromi E, Lovasz BD, Mandel M. Is early limited surgery associated with a more benign disease course in Crohn’s disease? World J Gastroenterol. 2013;19:7701-7710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Loftus EV Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Greenstein AJ, Sachar DB, Pasternack BS, Janowitz HD. Reoperation and recurrence in Crohn’s colitis and ileocolitis Crude and cumulative rates. N Engl J Med. 1975;293:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 196] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 490] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 7. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 8. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1620] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 9. | Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A nationwide analysis of changes in severity and outcomes of inflammatory bowel disease hospitalizations. J Gastrointest Surg. 2011;15:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Aratari A, Papi C, Leandro G, Viscido A, Capurso L, Caprilli R. Early versus late surgery for ileo-caecal Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Latella G, Cocco A, Angelucci E, Viscido A, Bacci S, Necozione S, Caprilli R. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis. 2009;41:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 13. | Gao X, Yang RP, Chen MH, Xiao YL, He Y, Chen BL, Hu PJ. Risk factors for surgery and postoperative recurrence: analysis of a south China cohort with Crohn’s disease. Scand J Gastroenterol. 2012;47:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Thirlby RC, Land JC, Fenster LF, Lonborg R. Effect of surgery on health-related quality of life in patients with inflammatory bowel disease: a prospective study. Arch Surg. 1998;133:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Casellas F, López-Vivancos J, Vergara M, Malagelada J. Impact of inflammatory bowel disease on health-related quality of life. Dig Dis. 1999;17:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, Present DH. Postoperative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Yoshida K, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, Yokoyama Y, Iimuro M, Takeda N, Kato K. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis. 2012;18:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients - a POCER study analysis. Aliment Pharmacol Ther. 2015;42:867-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | El-Hussuna A, Theede K, Olaison G. Increased risk of post-operative complications in patients with Crohn’s disease treated with anti-tumour necrosis factor α agents - a systematic review. Dan Med J. 2014;61:A4975. [PubMed] |
| 20. | Yang ZP, Hong L, Wu Q, Wu KC, Fan DM. Preoperative infliximab use and postoperative complications in Crohn’s disease: a systematic review and meta-analysis. Int J Surg. 2014;12:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Feagan BG, Yan S, Bala M, Bao W, Lichtenstein GR. The effects of infliximab maintenance therapy on health-related quality of life. Am J Gastroenterol. 2003;98:2232-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Feagan BG, Panaccione R, Sandborn WJ, D’Haens GR, Schreiber S, Rutgeerts PJ, Loftus EV, Lomax KG, Yu AP, Wu EQ. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 23. | Loftus EV, Feagan BG, Colombel JF, Rubin DT, Wu EQ, Yu AP, Pollack PF, Chao J, Mulani P. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103:3132-3141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 25. | Sakatani A, Fujiya M, Ito T, Inaba Y, Ueno N, Kashima S, Tominaga M, Moriichi K, Okamoto K, Tanabe H. Infliximab extends the duration until the first surgery in patients with Crohn’s disease. Biomed Res Int. 2013;2013:879491. [PubMed] |
| 26. | Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Siassi M, Weiger A, Hohenberger W, Kessler H. Changes in surgical therapy for Crohn’s disease over 33 years: a prospective longitudinal study. Int J Colorectal Dis. 2007;22:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Jones DW, Finlayson SR. Trends in surgery for Crohn’s disease in the era of infliximab. Ann Surg. 2010;252:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 547] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 30. | Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, Harmsen WS, Zinsmeister AR. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 251] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Loomes DE, Teshima C, Jacobs P, Fedorak RN. Health care resource use and costs in Crohn’s disease before and after infliximab therapy. Can J Gastroenterol. 2011;25:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |