INTRODUCTION
One to five percent of patients who receive radiotherapy as adjuvant or neoadjuvant therapy for pelvic malignancy will develop chronic haemorrhagic radiation proctitis (CHRP). In one of the recently published series, it was noted that 1319 patients received radiation for carcinoma of cervix over a period of 22 mo and 124 similar patients during the same period needed treatment for CHRP in the same centre[1]. The meaning of the above sentence shows the magnitude of the problem of CHRP. Newer methods of radiotherapy like three-dimensional conformal radiation therapy and intensity-modulated radiation therapy can use higher doses of radiation to the target tissues with less exposure to adjacent normal tissues. Protons and neutrons, so-called particle radiation, are also being tested but the long-term outcomes of these modalities are not known, and these are expensive. The use of brachytherapy is also found to be associated with fewer complications. Thus, the incidence of CHRP is related to the dose of radiation, the area of exposure, methods of delivery, the use of cytoprotective agents and other factors[2].
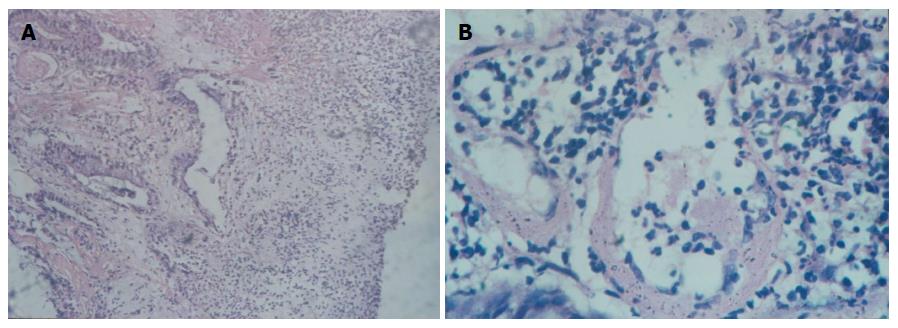
Because the rectum has a fixed position in the pelvis, it becomes more susceptible to radiation injury. Acute radiation injury of rectum occurs within three months of starting radiotherapy. It is an inflammatory process of rectal mucosa with a loss of microvilli, oedema, and ulceration. It is self-limiting and manifests as abdominal pain, tenesmus, diarrhoea, incontinence and urgency and resolves within three months[3]. Unlike acute radiation proctitis, chronic radiation proctitis takes a period of 3 mo after pelvic radiation, but usual median time is 8-12 mo. It can also continue from acute phase[3]. It is due to obliterative endarteritis, submucosal fibrosis, and neo-vascularization (Figure 1). Chronic radiation proctitis can present with rectal bleeding, tenesmus, mucus discharge, diarrhoea, incontinence, and urgency. It may be asymptomatic also. The diagnosis of radiation proctitis should be suspected if a patient presents with the above mentioned symptoms and gives a history of pelvic radiation. The diagnosis is confirmed by sigmoidoscopy or colonoscopy that shows pale, friable mucosa with telangiectasia. Rectovaginal, recto-urethral, recto-vesicular fistulizing disease is a late-presenting sign. There is no role of biopsy to confirm the diagnosis since it may produce complications.
Figure 1 Chronic radiation proctitis.
A: Chronic radiation proctitis (low power view). This picture shows the mucosa with severe oedema, non-specific inflammation, lymphocytosis, hyalinization in the stroma and fibrin thrombi in the postcapillary venules (Hematoxylin and Eosin stain, 10 ×); B: Chronic radiation proctitis (High power view). This picture shows two veins in the lamina propria, one with patchy occlusive fibrin thrombus. The wall shows thickening and hyalinization. A dense non-specific inflammation including few eosinophils also seen (Hematoxylin and Eosin stain, 40 ×).
There is no standard treatment for CHRP. However many treatments are available like amino salicylates, butyric acid enema, steroid enemas, formalin, argon plasma coagulation (APC), hyperbaric oxygen, radiofrequency ablation and even surgical therapy. The outcome of any of these medical and surgical treatment can be disappointing[1]. There are not many good-quality placebo-controlled trials.
In this study, our aim was to review the literature to see whether there is an improvement in the available evidence in comparison with previously published systematic reviews in treating patients with CHRP. The PubMed/Medline literature database was selectively searched for articles with the keywords “Proctitis/drug therapy”(Mesh) or “Proctitis/radiotherapy”(Mesh) or “Proctitis/surgery”(Mesh) and “radiotherapy”, “Management of CHRP”“Related Review articles”. In addition Google search and Google Scholar search was also made using key words “Radiation proctitis”“Formalin”“Endoscopic therapy”“APC”“Radiofrequency ablation”“cryotherapy”“Hyperbaric oxygen therapy” and “surgery”. The literature search was mostly limited to articles in English and human patients. No limitations for the year of publication were applied. All the studies that treated patients with rectal bleeding due to chronic radiation proctitis or CHRP were included in the review. Studies of patients treating acute radiation proctitis were excluded.
We could find 142 articles in total. After removing the duplicates and studies on acute radiation proctitis, there were about 86 articles. Out of these 86, 16 were further excluded because of various reasons such as anecdotal studies. Various studies that were found to be relevant are summarized below. Importance was given to randomized controlled clinical trials.
STUDIES USING ANTI-INFLAMMATORY DRUGS, STEROIDS, SUCRALFATE AND PENTOSAN POLYPSULPHATE
Sulfasalazine or 5-aminosalicylates, steroids are the drugs used initially for treating CHRP. Their mechanism of action is by inhibition of prostaglandin synthesis. It may also be due to inhibition of folate-dependent enzymes[4]. Sucralfate stimulates epithelial healing and forms a protective barrier[5]. Sucralfate is shown to be better than anti-inflammatory agents[6]. Pentosan polysulphate is similar to sucralfate. There are more than seven to eight publications using these drugs.
In a prospective double-blind, randomized controlled trial involving 37 consecutive patients with radiation-induced proctosigmoiditis[6], there were 36 females treated for cervical cancer and one male treated for prostate cancer. The mean duration after completion of the radiotherapy was 8.3 mo. These patients were randomized to receive either 3 g oral sulfasalazine plus 20 mg twice daily of rectal prednisolone enemas (group I, n = 18) or 2 g of rectal sucralfate enema plus oral placebo (group II, n = 19) for four weeks. These two groups were comparable with respect to demography, clinical symptoms and endoscopic staging of the disease. Patients in Sucralfate enema showed a better clinical response although endoscopically the response was not statistically significant. Follow-up was limited to 4 wk.
Rougier et al[7,8], in their randomized trial, compared betamethasone enema (5 mg bd) with hydrocortisone mousse (90 mg bd) and concluded that hydrocortisone group had a better outcome. There were 32 patients with CHRP in this study. The outcomes used were bowel activity, tenesmus, rectal bleeding and endoscopic grading. Follow-up was limited to 4 wk.
In another randomized study by Cavcić et al[9], compared combination of oral metronidazole (400 mg tds), mesalamine (1 g tds) and rectal betamethasone to oral mesalamine and rectal betamethasone and found that the rectal bleeding and ulcers were significantly lower in the metronidazole group. In this study, there were sixty patients randomized into either group. The efficacy of metronidazole was assessed on the basis of rectal bleeding, diarrhoea and proctosigmoidoscopy in all patients. The follow-up was up to 12 mo. Grigsby et al[10] prospectively showed the benefit of oral pentosan polysulphate given for a period of 1 year in 13 patients.
STUDIES USING SHORT-CHAIN FATTY ACID ENEMAS
Short-chain fatty acids (SCFA) stimulate the growth of colonic mucosa. The vasodilatation effect may improve the blood flow of colonic mucosa[11]. Butyric acid is the main SCFA. There are more than six studies using SCFA. Many of them are case series.
Two randomized studies showed non-significant improvement of symptoms and signs but both the studies were underpowered[12,13]. Talley et al[12] in their randomized double-blind placebo-controlled cross over trial of 15 patients treated one group with the butyric acid enema and another group with normal saline placebo. Symptoms score, endoscopic scores and even histology were compared.
Similarly, Pinto et al[13] in their randomized prospective double blind controlled trial of 19 patients treated one group with SCFA enema and another group with placebo. In this study apart from symptoms and endoscopic features, biopsies for mucosal DNA and protein content were also measured. Patients were followed up to 6 mo.
Though we were treating patients with CHRP in our institute since 1985, study on chronic haemorrhagic proctitis were started in 1999. The first study on CHRP in our institute, done by Senthil Kumar et al[14] in 2001, compared sucralfate-steroid enema (25 mg of prednisolone and 1 g of sucralfate twice daily for 14 d) with butyric acid retention enema (60 mL containing 40 mmol of butyric acid twice daily for 14 d) in a double-blind randomized controlled trial. There were thirty patients randomly allocated. They were followed up to 4 wk. Outcomes were measured by the improvement in the colonoscopic grading of severity and clinical symptoms. Histopathological improvements were also compared by taking the biopsy before and after treatment. The conclusion was that both the methods of treatment were equally effective since there was relief of symptoms of radiation proctitis in both the methods of treatment without improvements in endoscopic scores or histology. However, the sucralfate-steroid enema was easier to prepare[14]. No toxicities were reported in any of these studies.
STUDIES USING FORMALIN THERAPY
Formalin scleroses and seals fragile neovasculature in tissues damaged due to radiation and prevents further bleeding. In 1986, Rubinstein was the first to use formalin for a CHRP patient to get a good response[15]. Following this, there are several reports in the literature[16-30]. But the majority are retrospective in nature, a few are prospective studies. The technique and the concentration of formalin used in these studies also differ. The two main methods of using it are 4% solution as irrigation or as soaks. There are reports of using 10% solution of formalin also[31]. There are four Randomized trials using formalin for CHRP.
Ours is one of the first published randomized trial comparing the efficacy of the 4% formalin dab with Sucralfate-steroid retention enema (100 mg of prednisolone and 1 g sucralfate in 100 mL of normal saline twice daily for 14 d)[1]. In this study, 102 patients were randomly allocated to either of the treatment arms. This study objectively assessed the symptoms scores using the radiation proctopathy system assessment scale (RPSAS) and also the sigmoidoscopic grade (Modified Chi grading) before and after treatment and found that Formalin dab is superior to sucralfate-steroid enema in treating CHRP involving only the rectum. It was also observed that a single session of formalin dab can effectively treat CHRP in 90% of the patients, and multiple sessions could effectively treat 99% of the patients whereas sucralfate-steroid enema was effective only in 75% of patients. These patients were followed up to 9 mo. There was no complications or toxicity.
Following this Yeoh et al[32] showed in their randomized study that APC and topical formalin had comparable efficacy in the durable control of rectal bleeding associated with chronic radiation proctitis but had no beneficial effect on anorectal dysfunction. In this study thirty patients were randomized into each group. Anorectal symptoms, (modified LENT-SOMA questionnaire) anorectal manometry and anorectal morphology by endorectal ultrasound were assessed before and after treatment.
Guo et al[33] in their randomized trial showed that 10% formalin is associated with complications and 4% formalin should be the choice for treating CHRP. In this study 122 patients were randomized into 4% or 10% formalin application. Outcomes were compared with symptoms score and rectoscope scores. Follow-up was up to 1 year. Wong et al[34] from their prospective database, after a decade of experience of treating patients with radiation proctitis, have shown that formalin is more effective than APC in treating patients with CHRP. APC has the potential to complement topical formalin application and can be used to treat the proximal and distal rectum concurrently.
The contrary report has been published by Alfadhil et al[35] in their retrospective comparative study of 22 patients who received formalin application or APC. Improvement in Hb% was used to assess the outcome. The severity of the proctitis was not assessed before the treatment. The lag time between radiation and endoscopic treatment was not known. The study was underpowered, and the groups were not comparable. The details of the adverse events not mentioned. They concluded that APC is more effective than formalin and has less adverse effects.
Sahakitrungruang et al[36] in their randomized controlled trial comparing colonic irrigation with oral antibiotics administration vs 4% formalin application for treatment of CHRP have shown that the former method is better than 4% formalin application. Fifty patients were randomly allocated to each arm. Daily self-administered colonic irrigation of 1 L tap water and a 1-wk period of oral antibiotics-ciprofloxacin and metronidazole were given in one arm. Four percent formalin application for 3 min was done in another arm. Patient’s satisfaction was surveyed. The limitation was that the study was a 2-armed design without a crossover trial. Hence, it could not illustrate whether the antibiotics and irrigation were equally important. Some of the adverse events noted in the literature regarding the use of formalin for CHRP are the rectal stricture, worsening of incontinence, anococcygeal pain, and formalin colitis[24,30].
STUDIES USING THERMAL COAGULATION THERAPY
Endoscopic coagulation with a variety of devices has been reported to be effective for CHRP[7]. The technique involves coagulation of a bleeding point rather than the entire friable mucosa. Several treatment sessions are often required[7]. The modalities include heater probe, bipolar Electrocoagulation, neodymium:yttrium-aluminium-garnet (Nd:YAG) laser, potassium titanyl phosphate (KTP) laser, argon laser and APC. Simple heater probe and APC are preferred for their better safety profile[37].
Both the heater probe and bipolar cautery are contact probes. The heater probe has a Teflon-coated heating element at its tip that delivers standardized energy over set times. Bipolar electrocautery probe has a pair of electrodes at its tip through which current is passed using the tissue for conduction[38]. Jensen et al[39] in his randomized study showed that 21 patients treated either with a heater probe, or bipolar cautery showed benefits without much difference between the two modalities[39]. A mean of four sessions was needed in each arm during treatment in this study. Patients were followed up to one year. The increase in haematocrit, endoscopic resolution, and patient satisfaction were compared. No complications were noted.
Nd:YAG laser is the first endoscopic laser used for treating CHRP. Some of the complications reported with this are transmural necrosis, fibrosis, necrosis, stricture formation and recto-vaginal fistula. Nd:YAG laser use for CHRP has declined due to several reasons, firstly its cost; second, the need to aim directly at telangiectasias and the possibility of severe endoscopic damage if the laser strikes the endoscope in retroflection[40]. Taylor et al[41] used KTP laser for treating 26 patients with bleeding secondary to CHRP using 4-10 W and a median of two sessions. They reported a symptomatic improvement in 65% patients while there was no change in 7 (30%), and symptom like hematochezia increased in 1 (5%). Similarly, there are only case series using argon laser for treating CHRP.
There are more than 15 published reports of APC for CHRP. Many are retrospective studies, and some of them are prospective case series. Many of the series report unsuccessful medical treatment before going for APC. In APC bipolar diathermy current is applied using inert argon gas as a conducting medium. It can be applied tangentially and radially.
Karamanolis et al[42] showed in their prospective study treating more than 56 patients, that APC was successful in all patients with mild and in almost all patients with moderate CHRP. In contrast, APC failed in 50% of patients, wherever the presence of severe mucosal damage was present. The grading of severity was based on endoscopic criteria taking into consideration telangiectasia distribution and surface area involved. For APC application, a 2.3 mm diameter front firing APC probe inserted through the working channel of the flexible sigmoidoscope was used. The argon flow rate and the electrical power were set at 2 L/min and 40 W, respectively. Patients were followed up for a mean of 17 mo. Patients required 1-2 sessions of APC for mild proctitis while patients with moderately to the severe form required a statistically significantly higher number of APC sessions. In cases of severe and diffuse involvement of the rectum, multiple treatments sessions are required, and success is less certain as shown by other reports also[43-51]. In many of these series, the response is objectively scored using bleeding severity score, haematological parameters, and endoscopic scores.
Chruscielewska-Kiliszek et al[52] in their randomized, double-blind trial comparing oral sucralfate or placebo following APC for CHRP have shown that additional sucralfate treatment after APC did not influence the clinical or endoscopic outcomes. One hundred and seventeen patients completed the treatment protocol, 57 in the sucralfate group and 60 in the placebo group. Patients were graded clinically and endoscopically according to the Chutkan and Gilinski scales before and at 8 and 16 wk after initial APC treatment (1.5-2 L/min, 25-40 W) and after 52 wk (clinical only)[52]. Complications (1%-15%) following APC, such as pain, ulceration, perforation, explosion, extensive necrosis and rectal stricture have been cited in the literature[42].
STUDIES USING RADIO-FREQUENCY ABLATION
There are more than five reports of case series and retrospective studies using radio-frequency ablation (RFA) for CHRP. Many case series have shown, using BARRx Halo90 electrode catheter that was fit on the distal end of the flexible sigmoidoscope, an energy density of 12 J/cm2 at a power density of 40 W/cm2, hemostasis could be obtained after 1 to 2 sessions[53-56].
Several benefits RFA have been claimed, these include squamous re-epithelialization, lack of stricturing and ulceration. Using RFA much broader area of tissue can be treated simultaneously compared to the point by point approach by other methods[37]. The radio-frequency unit is mobile and can be used in different rooms of an endoscopy unit. Zhou et al[54] have used real-time endoscopic optical coherence tomography (EOCT) to visualize epithelialization and subsurface tissue microvasculature pre- and post-treatment RFA in their case series and have shown the potential of EOCT for follow-up assessment of endoscopic therapies.
STUDIES USING CRYOABLATION
Cryoablation is similar to APC and involves the non-contact application of liquid nitrogen or carbon-dioxide to tissues for superficial ablation. It is possible to treat a larger surface area like in RFA. Its effect is due to ischemic necrosis which can be immediate or delayed.
There are only case series of 20 patients. During cryoablation, a decompressive rectal tube has to be inserted because of the risk of over insufflation and perforation. Cryotherapy units are less mobile. Unlike in Radiofrequency ablation, the depth of tissue penetration may be more here. This may lead to strictures. However, colonic lavage is not necessary since there is no risk of explosion. The number of required sessions range from one to four[57-59].
STUDIES USING HYPERBARIC OXYGEN THERAPY
There are more than 12 published studies using hyperbaric oxygen therapy (HBOT) for CHRP. New reports have started appearing in the literature regarding the efficacy of HBOT.
Clark et al[60] in their randomized controlled double-blind crossover trial (150 patients) with a long-term follow-up, up to 5 years, showed that in patients with refractory CHRP, HBOT had a significant healing response. Primary outcome measures involved were the late effect in normal tissue-subjective, objective, management, analytic (SOMA-LENT) score and standardized clinical assessment. The secondary outcome was the change in the quality of life[60].
In one of the largest Australasian study using HBOT for chronic radiation injuries, Tahir et al[61] showed a clinical response rate for CHRP of 95%, where around half of the cases had a durable major response, with some patients experiencing symptom relief lasting as long as seven years.
At pressure greater than atmospheric pressure and using 100% oxygen, HBOT has an angiogenic effect and has been shown to cause an eight to nine-fold increase in the vascular density of soft tissues over air-breathing controls[7]. HBO acts to stimulate collagen formation and re-epithelialization. There is no uniformity in the methods of treatment using HBOT[42,61-65]. Although it can be perceived from the studies that HBOT is useful in refractory radiation proctitis, there is marked variation between the studies. There are no major adverse effects. Minor adverse event recorded is transient aural barotrauma. The reported number of HBOT sessions for a successful treatment range from 12 to 90. The cost of HBOT is high, and hence, it is not widely applicable.
Other interventions
Oxidative stress is thought to be one of the mechanisms in the development of chronic radiation proctitis and antioxidants have been used to treat CHRP. Use of vitamin C and E have been reported. Kennedy et al[66] treated twenty consecutive patients with CHRP. They used a combination of vitamin E at a dose of 400 IU tid and vitamin C at a dose of 500 mg tid. They assessed the response by symptom index and lifestyle questionnaire. A good number of study patients in the study seem to benefit. This pilot study was not studied further.
Retinol palmitate (vitamin A) has been shown to increase wound healing because of increased collagen cross-linking. This has been used in a randomized study to show improvement of symptoms of chronic radiation proctopathy by Ehrenpreis et al[67]. They randomized 19 patients, 10 patients to retinol palmitate group and nine to the placebo group. Five placebo nonresponders were crossed over to the retinol palmitate. The RPSAS scores before and every 30 d for 90 d were measured. The definition of response was a reduction in two or more symptoms or by at least two RPSAS[67]. There was a significant improvement in symptoms in the treatment group compared with the control and also when the controls were crossed over to treatment. But the study was underpowered.
Surgical interventions
Surgery is the last resort in patients with CHRP. Around 10%-25% of patients with CHRP finally need surgery[68]. Intractable bleeding, perforation, stricture, and fistula are some of the indication for surgery in patients with chronic radiation proctitis. There are case reports of non-surgical dilatation for strictures for this condition[69]. Significant improvement of bleeding by diversion has been shown by one of the retrospective study[70]. Fistula with the adjacent structures may need resection or resection with reconstruction with a diverting stoma. Whenever surgical treatment became a necessity, studies report poor outcomes with high complications (15%-80%) and mortality (3%-9%)[70-73]. Since nonoperative interventions are commonly used nowadays in managing patients with CHRP, There are no recently published series on surgical interventions on this issue.
Discussion
Evidence-based medicine requires the systematic and critical evaluation of published and unpublished trials[74]. In 2002, when Denton et al[7] first published their systemic review of the non-surgical intervention of late radiation proctitis, they could identify only six randomized controlled trials. The majority of the evidence available was either one individual’s or one center’s experience with a specific intervention without comparison to a control or another agent. This is probably due to the low incidence of chronic radiation proctitis in the majority of centers and the difficulties that co-exist in compiling a series large enough to be randomized between therapies[34].
Thirteen years later we could identify a total of 14 randomized controlled trials treating 804 patients with CHRP. In many of these studies, we could get the details of the reason for radiation therapy and the dosage. The diagnosis was based on the history and the endoscopic findings. At present, there is no validated score for CHRP, which can be used universally for grading the severity. There can be the inter-observer difference of the same findings. Tissue biopsy may not be conclusive. Patients may not tell their exact symptoms unless directed questions are asked. The severity of the radiation proctitis was graded in many of the studies objectively using symptoms score like RPSAS[1,67] or LENT-SOMA scale[32,60] and intraluminal findings by the sigmoidoscopic or colonoscopic grade (modified Chi grading[1] or Chutkan and Gilinski scales[52]). But different studies used different severity scores and hence the inter-institutional comparison of data is still difficult. The same is true with the outcome measures. Yeoh et al[32] have tried to see the rectal functions as well as morphology by using anorectal manometry and endorectal ultrasound. Zhou et al[54] have shown the most efficient objective assessment of the response to treatment by using EOCT. There were only a few studies that surveyed the quality of life following treatment[36,60]. However, unlike the previous studies, follow-up of recent studies is fairly long and is usually more than 9 to 12 mo[1,33,36,52,60]. With these randomized trials it is possible to say that there is evidence to make the following judgments: (1) Sucralfate enema appears to have a better effect than anti-inflammatory agents; (2) Anti-inflammatory drugs appear to have a better effect if used with oral metronidazole; (3) Rectal hydrocortisone appears to have a better effect than rectal betamethasone; (4) Sucralfate-steroid retention enema and short chain fatty acid enema are both equally but moderately effective in treating CHRP, but sucralfate-steroid enema is easy to prepare; (5) Four percent formalin is more effective than sucralfate-steroid retention enema and can be effective in 99% of the patients of CHRP; (6) Four percent formalin should be preferred over 10% formalin in treating patients with CHRP since 10% formalin is likely to cause adverse events; (7) Heater probe and bipolar cautery are equally effective in treating patients with CHRP; (8) Both APC and formalin don’t improve the rectal dysfunction but only stop the bleeding; (9) Both APC and formalin are equally effective, but formalin may be better in severe disease; (10) Additional oral treatment after APC will not improve the outcomes; (11) Radiofrequency ablation is a promising upcoming modality of treating CHRP but more robust data in the form of randomized trials needed; (12) HBOT is the only treatment modality, currently, which addresses the underlying problem and effective in treating CHRP patients but is costly and available in a few centers; (13) Vitamin A and other modalities have to be kept in mind while treating these patients since some report shows its efficacy. Further trials and robust data needed to show its efficacy; and (14) Surgical intervention is to be kept as a last resort in patients not responding to any of the methods described above.
Looking at the available evidence, it is clear that there is some improvement in the methodology of these studies. There is an objective assessment of symptoms and signs and also the objective assessment of the outcomes in some of these studies. The major drawback is that the objective assessment is not uniform, different studies using different scores. Also, not much importance is given to the quality of life assessment following treatment. It has been felt by the previous reviewers that one study, even if well conducted, will not be able to modify the changes in practice[7]. It has been felt by Denton et al[7] that in order to increase recruitment to trials a national registry of CHRP cases would facilitate multicenter trials with uniform entry criteria, uniform baseline and uniform therapeutic assessments providing standardized outcome data[75].
Limitations of this review: The search was limited to PubMed/Medline, Google and Google Scholar and was not complete and was limited to English language journals only. Individual authors were not contacted.
CONCLUSION
Based on this evidence, it can be concluded that the first line treatment of a patient with CHRP, the most effective way of treating CHRP, should be 4% formalin application. Since it is cheap, easily available, can be applied easily and effective in 99% of the patients. If the radiation proctitis extends beyond rectum, then APC will be a better alternative. Alternatively, both formalin and APC can be used as complementary methods. Those patients who are refractory to formalin or APC may be referred for treatment with HBOT. In centers where radiofrequency ablation is available, further randomized studies should be done to see the efficacy of it in treating patients with CHRP. Those patients with CHRP who do not respond to any of the modality may need surgery in the form of diversion colostomy. Those patients with CHRP presenting with complications like stricture, fistula or other complications like obstructions may need surgery at presentation.
Manuscript source: Invited manuscript
P- Reviewer: Jongen J, Linard C, Pigo F S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK