Published online May 27, 2016. doi: 10.4240/wjgs.v8.i5.389
Peer-review started: July 5, 2015
First decision: September 17, 2015
Revised: February 8, 2016
Accepted: March 4, 2016
Article in press: March 9, 2016
Published online: May 27, 2016
Processing time: 323 Days and 23.6 Hours
AIM: To identify therapeutic agents for the prophylaxis of gastrointestinal anastomotic leakage (AL) under complicated conditions.
METHODS: The PubMed and EMBASE databases were searched for English articles published between January 1975 and September 2014. Studies with the primary purpose of improving anastomotic healing in the colon or rectum under complicated preoperative and/or intraoperative conditions were included. We excluded studies investigating the adverse effects or risk assessment of an active intervention. Furthermore, investigations of biophysical materials, sealants, electrical stimulation and nutrients were excluded. The primary study outcome was biomechanical anastomotic strength or AL. The meta-analysis focused on therapeutic agents that were investigated in one animal model using the same outcome by at least three independent research groups.
RESULTS: The 65 studies included were divided into 7 different complicated animal models: Bowel ischemia, ischemia/reperfusion, bowel obstruction, obstructive jaundice, peritonitis, chemotherapy and radiotherapy. In total, 48 different therapeutic compounds were examined. The majority of investigated agents (65%) were reported as beneficial for anastomotic healing. Twelve of the agents (25%) were tested more than once in the same model, whereas 13 (27%) of the agents were tested in two or more models of complicated healing. Two therapeutic agents met our inclusion criteria for the meta-analysis. Postoperative hyperbaric oxygen therapy significantly increased anastomotic bursting pressure in ischemic colon anastomoses by a mean of 28 mmHg (95%CI: 17 to 39 mmHg, P < 0.00001). Granulocyte macrophage-colony stimulating factor failed to show a significant increase in anastomotic bursting pressure (95%CI: -20 to 21 mmHg, P = 0.97) vs controls in experimental chemotherapeutic models.
CONCLUSION: This systematic review identified potential therapeutic agents, but more studies are needed before concluding that any of these are useful for AL prophylaxis.
Core tip: Anastomotic leakage is a challenging complication after colorectal surgery. Although many pharmaceutical compounds have the potential to improve anastomotic healing, none has reached the clinical setting. This study reviewed 65 experimental studies investigating 48 different therapeutic agents for the improvement of anastomotic healing under complicated conditions due to ischemia, ischemia/reperfusion, obstructive bowel, obstructive jaundice, peritonitis, chemotherapy or radiotherapy. Of the 31 agents reported to enhance anastomotic healing, one was subjected to a meta-analysis. Hyperbaric oxygen therapy significantly improved anastomotic healing in rat models complicated by bowel ischemia. Further exploration is needed to define agents that reduce AL in high-risk patients.
- Citation: Nerstrøm M, Krarup PM, Jorgensen LN, Ågren MS. Therapeutic improvement of colonic anastomotic healing under complicated conditions: A systematic review. World J Gastrointest Surg 2016; 8(5): 389-401
- URL: https://www.wjgnet.com/1948-9366/full/v8/i5/389.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i5.389
Colorectal surgery with construction of a primary anastomosis is performed for conditions such as cancer, diverticulitis, ulcerative colitis, ischemia or stoma reversal.
Despite improvements in preoperative management and surgical techniques, anastomotic leakage (AL) remains a major complication. The incidences of AL after colonic resection and rectal resection are 3%-7% and 10%-20%[1-3], respectively. AL is associated with increased risk of morbidity, short-term mortality, permanent ostomy, tumor recurrence and a diminished overall long-term survival[2,4-8].
In animal models of anastomotic healing, anastomotic bursting pressure (BPR) and anastomotic breaking strength (BST) are the most common surrogate outcomes of anastomotic healing. BPR reflects the resistance to increased intraluminal pressure, whereas BST reflects the increased longitudinal load. The collagen concentration is important for anastomotic integrity and declines to a minimum 3 d after the construction of colonic anastomoses under normal healing conditions[9,10].
Previous studies have identified several local and systemic factors with deleterious effects on anastomotic wound healing, including ischemia[11,12], reperfusion[13-15], bowel obstruction[16], obstructive jaundice[17], peritonitis[11,16,18,19], chemotherapy[20] and radiotherapy[3,20]. Reperfusion after intestinal ischemia provokes local and systemic inflammatory responses[13-15], and ischemia ultimately leads to tissue necrosis and bowel perforation[21,22]. Acute bowel obstruction is associated with ischemia, inflammation and loss of collagen in the colonic wall[9,23,24]. Obstructive jaundice compromises systemic immune functions[17]. Impaired collagen synthesis is observed in peritonitis[25] and with the use of chemotherapeutic agents[26]. Preoperative radiotherapy, which is used to downsize rectal tumors, induces inflammation[3,27]. Despite the well-known risk of compromised anastomotic healing under these conditions, surgical resection and construction of a primary anastomosis are pivotal in the treatment algorithm.
A recent meta-analysis identified seven compounds, including iloprost, tacrolimus, erythropoietin (EPO), growth hormone (GH), insulin-like growth factor-1 (IGF-1), hyperbaric oxygen therapy (HBOT) and synthetic inhibitors of matrix metalloproteinases (MMPs), all of which have the potential to improve anastomotic healing under non-complicated conditions[28]. Several compounds have also been tested in different experimental models of complicating conditions[29].
The aim of the present systematic review was to identify therapeutic agents that are potentially capable of abolishing or reducing the deleterious effects on anastomotic healing caused by ischemia, ischemia/reperfusion (I/R), obstructive bowel, obstructive jaundice, peritonitis, chemotherapy or radiotherapy.
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[30].
The PubMed and EMBASE databases were searched for articles published between January 1975 and September 2014 using the following syntax: ((Surgical anastomo*) OR (intestinal anastomo*) OR (anastomo* AND leak*) OR (anastomo* AND dehiscence) OR (surgical wound dehiscence) OR (anastomo* AND failure) OR (anastomo* AND rupture)) AND ((colorectal surgery) OR (surgical anastomo*) OR (colo* AND surgery) OR (rect* AND surgery) OR (large intestine and surgery) OR (colorectal resection)) AND ((burst* pressure) OR (breaking strength) OR (anastomo* AND strength) OR (wound rupture) OR (biomechanical strength) OR (mechanical strength) OR (wound healing) OR (autopsy) OR (anastomo* AND leak*)) AND (((peritonitis) OR (infection) OR (sepsis)) OR ((ischemia) OR (hypoperfusion)) OR ((ileus) OR (bowel obstruction) OR (large bowel obstruction) OR (intestinal obstruction)) OR ((radiation) OR (radiotherapy) OR (radiochemotherapy) OR (chemotherapy))).
Cross-references from the included studies were manually reviewed.
Titles of the articles identified in the search were reviewed, and potentially relevant abstracts or full-text articles, if necessary, were assessed for eligibility. Two or more authors decided whether a study qualified for inclusion, and disagreements were solved by discussion among the four authors.
The abstracted data included the complicated animal model used, the investigated compound, the time of administration, the species, the gender, the sample size, the dosage, the route, the day of anastomotic testing, the primary outcome and the effects on BPR, BST or AL of the compound investigated.
Missing data were gathered by contacting the authors.
English publications with the primary aim of investigating the potential beneficial properties of a pharmacological agent to improve anastomotic healing during complicated conditions were included. Studies on animals or humans with colo-colonic or colorectal anastomoses without a protecting ostomy reporting BPR, BST and AL relative to a proper control group were included.
Studies with the aim of clarifying adverse effects or risks of a therapeutic agent on anastomotic healing in complicated conditions were excluded. Likewise, studies on the effects of electrical stimulation, mechanical enforcement, such as biofragmentable anastomotic rings, endoluminal prosthesis/tube or amniotic membranes, together with sealants, such as fibrin glue, cyanoacrylates or collagen matrix bound coagulation factor sealants, and nutrients were also excluded.
Compounds investigated in one complicated animal model by at least three independent research groups using the same primary outcome were subjected to a meta-analysis. For these analyses, Review Manager (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used. Pooled estimates were calculated using the inverse-variance weighting method with the DerSimonian-Laird random-effects model. Heterogeneity among the studies was determined using I2 tests. The level of statistical significance was 0.05.
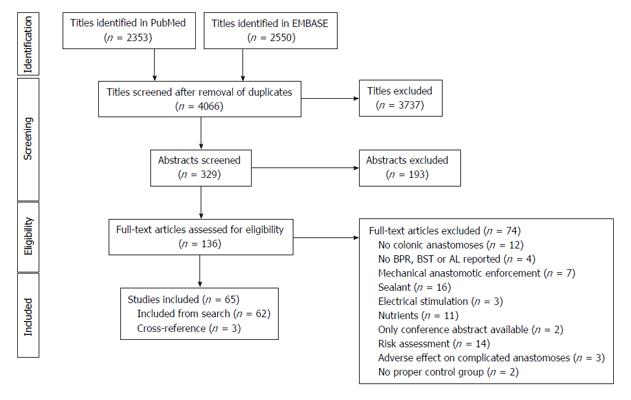
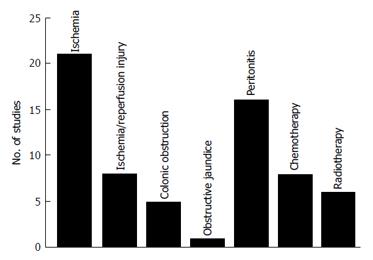
A total of 65 studies were included in the study (Figure 1). These studies were divided into 7 different animal models (Figure 2): Bowel ischemia models (n = 21) in rats (n = 20) and dogs (n = 1), I/R injury models (n= 8) in rats, models of colonic obstruction (n = 5) in rats (n = 4) and guinea pigs (n = 1), an obstructive jaundice model in the rat (n = 1), models of peritonitis (n = 16) in rats (n = 15) and mice (n = 1), chemotherapeutic models (n = 8) in rats and irradiation models (n = 6) in rats (n = 5) and pigs (n = 1). The reported outcomes were BPR (n = 62), BST (n = 4) and AL (n = 5). More than one outcome was applied in 6 studies. No human studies were retrieved by our search criteria.
Forty-eight different compounds were identified; 12 (25%) compounds were tested more than once in the same model, and 13 (27%) were tested in more than one complicated model. Enhancement of anastomotic healing was reported for 31 (65%) of the compounds; a non-significant effect was reported for 7 (15%) of the compounds, inconsistent results were reported for 9 (18%) different compounds and 1 (2%) compound was found to be detrimental to anastomotic healing.
Twenty-two different compounds were tested in models of intestinal ischemia (Table 1). Experimentally, ischemia in the anastomotic segment was induced by ligation[21,31-33] or coagulation[34] of vessels in the mesocolon. The anastomosis was then constructed in the ischemic segment during the same surgical procedure.
| Ref. | Compound | Time of administration | Species | Sex | Sample size1 | Dosage | Route | Test | Test day | Effect2 |
| Yagci et al[33] | HBOT | Preoperative | Rat | M | 20 | BPR | 5 | NS | ||
| Postoperative | 20 | ↑ 23 | ||||||||
| Preoperative and postoperative | 20 | ↑ 37 | ||||||||
| Hamzaoğlu et al[31] | HBOT | Postoperative | Rat | M | 16 | BPR | 4 | ↑ 32 | ||
| Guzel et al[35] | HBOT | Postoperative | Rat | F | 20 | BPR | 4 | ↑ 50 | ||
| HBOT + β-1,3-glucan | 20 | 203 | IP | ↑ 67 | ||||||
| β-1,3-glucan | 20 | 203 | IP | ↑ 50 | ||||||
| Kemik et al[36] | HBOT | Postoperative | Rat | F | 20 | BPR | 4 | ↑ 80 | ||
| HBOT + LMWH | 20 | 14 | SC | ↑ 67 | ||||||
| LMWH | 20 | 14 | SC | NS | ||||||
| Adas et al[21] | VEGF-A plasmid | Intraoperative | Rat | M | 40 | 0.0013 | LO | BPR | 4 | ↑ 16 |
| FGF-2 plasmid | 0.0013 | ↑ 14 | ||||||||
| VEGF-A + FGF-2 plasmids | 0.0013 + 0.0013 | ↑ 38 | ||||||||
| Saribeyoğlu et al[38] | PDGF-BB | Intraoperative | Rat | M | 20 | 1253 | LO | BPR | 4 | ↑ 8 |
| Yarimkaya et al[32] | GH | Preoperative and postoperative | Rat | M | 28 | 15 | SC | BPR | 3/7 | ↑ 87/↑ 32 |
| Nandrolone | Preoperative | 28 | 24 | IM | ↑ 55/NS | |||||
| Tasdelen et al[39] | Leptin | Postoperative | Rat | N/A | 20 | 0.0014 | IP | BPR | 7 | ↑ 27 |
| Parra-Membrives et al[34] | Pentoxifylline | Postoperative | Rat | M, F | 38 | 504 | IP | BPR/BST | 8 | ↑ 74/↑ 81 |
| Sümer et al[40] | Pentoxifylline | Postoperative | Rat | M, F | 20 | 504 | IP | BPR | 5 | NS |
| Vinpocetine | 20 | 14 | NS | |||||||
| Karatepe et al[41] | Adrenomedullin | Postoperative | Rat | F | 32 | 0.0023 | SC | BPR | 3/7 | ↑ 5/↑ 20 |
| Cetinkaya et al[43] | Bostentan | Postoperative | Rat | F | 20 | 3.54 | IP | BPR | 6 | ↑ 46 |
| Garcia et al[22] | Allopurinol | Preoperative and postoperative | Rat | M | 20 | 504 | PO | BPR | 4 | ↑ 74 |
| Adas et al[44] | MSCs | Intraoperative | Rat | M | 40 | 0.56 | LO | BPR | 4/7 | ↑ 110/↑ 86 |
| Adas et al[45] | MSCs | Postoperative | Rat | M | 40 | 0.56 | iv | BPR | 4/7 | ↑ 42/NS |
| Dinc et al[46] | GM-CSF | Intraoperative | Rat | M | 72 | 0.0504 | LO | BPR | 3/7 | ↑ 30/↑ 26 |
| Ikeda et al[47] | Prostacyclin analogue (OP-41483) | Intraoperative and postoperative | Dog | M, F | 107 | 0.000044 | iv | AL | 3 | NS |
| 188 | ↓ 100 | |||||||||
| 129 | NS | |||||||||
| Cohen et al[48] | Neomycin + erythromycin | Preoperative | Rat | N/A | 12 | 203 | PO | AL | 7 | ↓ 83 |
| Clindamycin + gentamicin | 12 | 154 + 24 | iv | NS | ||||||
| Karataş et al[49] | Amelogenin | Intraoperative | Rat | M | 16 | N/A | LO | BPR | 4 | ↑ 25 |
| Irkorucu et al[50] | Sildenafil | Postoperative | Rat | M | 27 | 104/204 | PO | BPR | 4 | NS/NS |
| Coneely et al[51] | Compound 48/80 | Preoperative | Rat | M | 20 | 14 | iv | BPR | 4 | NS |
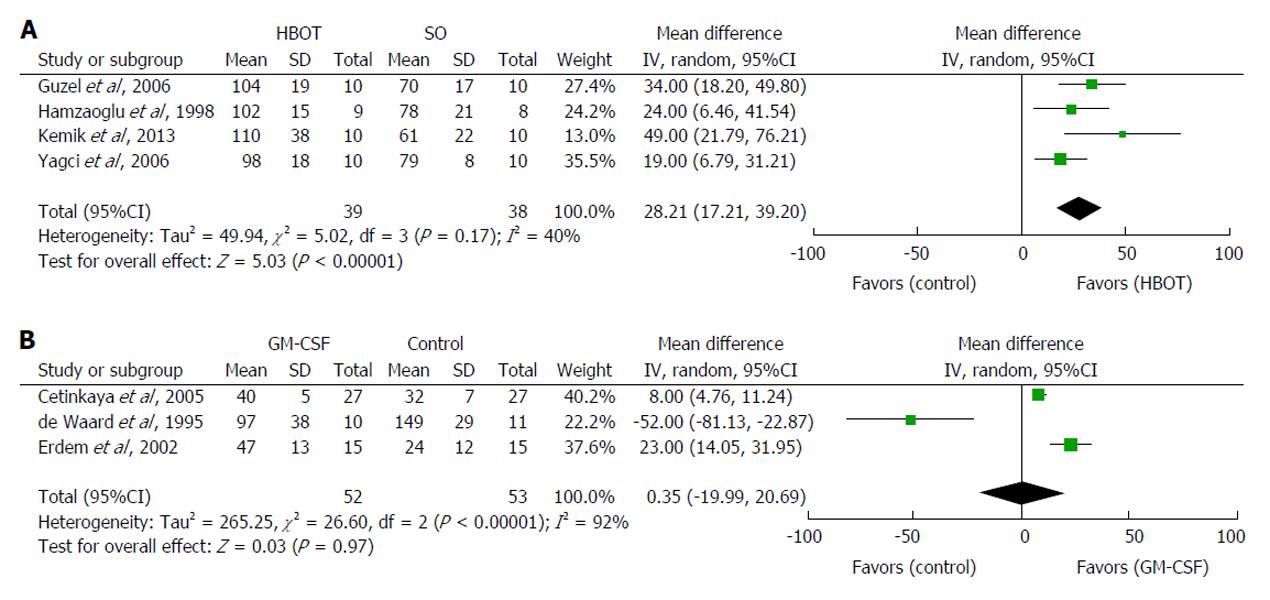
Four studies tested the effect of postoperative HBOT in rats[31,33,35,36]. The meta-analysis demonstrated that HBOT significantly increased anastomotic BPR by a mean 28 mmHg (95%CI: 17 to 39 mmHg, P < 0.00001) compared with controls (Figure 3A). The inconsistency between studies was moderately large (I2 = 40%). HBOT increases tissue oxygenation[31,33,35,36], which may explain the elevated hydroxyproline concentration in the anastomosis[33,35]. HBOT was ineffective when only administered preoperatively[33]. The possible adverse effects of HBOT are oxygen toxicity, air embolization and pneumothorax[31,33,35].
Guzel et al[35] found that rats receiving a postoperative intraperitoneal injection of β-1,3-glucan together with HBOT increased BPR by 67% compared with 50% for HBOT alone. β-1,3-glucan alone also significantly improved BPR[35]. Supplementing postoperative HBOT with low molecular weight heparin (enoxaparin) had no further effect on BPR despite increasing neovascularization in the anastomotic area[36]. Enoxaparin did not significantly improve BPR[36].
Growth factors and hormones are pivotal in wound healing[37]. Vascular endothelial growth factor (VEGF)-A and fibroblast growth factor (FGF)-2 plasmids were injected directly into the anastomotic tissue intraoperatively. The gene therapy increased VEGF and FGF-2 protein levels, BPR, angiogenesis, fibroblast activity and collagen deposition[21]. VEGF-A and FGF-2 plasmids combined were more effective than the growth factor genes administered individually[21]. The possibility of synergism between growth factors other than these two would be worthwhile to explore[21]. Platelet-derived growth factor (PDGF)-BB in a gel applied once to the suture line immediately after construction of the anastomosis increased BPR on day 4[38]. The mechanism remains elusive because PDGF-BB did not increase hydroxyproline as an indicator of collagen levels[38]. The anabolic hormones, GH and nandrolone, enhanced early anastomotic healing, presumably by increasing IGF-1 and structural proteins[32].
Although the main role of leptin is regulation of body weight and energy expenditure, in vitro studies have indicated a direct mitogenic effect of leptin on colonic epithelial cells[39]. Intraperitoneal leptin also increased the anastomotic strength of right-sided colon anastomoses in rats[39].
Pentoxifylline enhanced anastomotic BPR on day 8[34], but not on day 5[40].
The vasoactive adrenomedullin increased BPR and hydroxyproline levels on postoperative days 3 and 7[41]. Furthermore, adrenomedullin treatment decreased anastomotic tissue concentrations of tumor necrosis factor-α and interleukin-6[41]. Increased vascularization and less oxidative damage of the anastomoses were observed with adrenomedullin[41]. Adrenomedullin causes significant hypotension that may impair the colonic blood flow[41]. Another caveat is that adrenomedullin may induce neoplasia[41,42].
The beneficial effects of the endothelin receptor antagonist, bosentan, on anastomotic healing were possibly due to the increased blood flow and increased hydroxyproline level in the anastomotic area[43]. Bosentan significantly reduced adhesion formation[43].
Allopurinol reduced the induced superoxide anion production in ischemic anastomoses and increased the hydroxyproline levels[22].
Allogeneic mesenchymal stem cells (MSCs) derived from bone marrow of rats were cryopreserved. The cells were thawed and injected (1 × 106 viable MSCs) into newly constructed anastomoses in ischemic rat colon. This cell therapy resulted in enhanced BPR on both day 4 and day 7[44], whereas systemically applied MSCs resulted in a significant effect on day 4 only[45].
Locally applied granulocyte macrophage-colony stimulating factor (GM-CSF) enhanced anastomotic BPR on days 3 and 7[46].
A study on the effect of a prostacyclin analogue (OP-41483) on AL was undertaken in dogs with colonic ischemia of variable severity[47]. Colonic ischemia was induced by devascularization of marginal vessels resulting in slight (40%-60% decrease in colonic blood flow), moderate (60%-80%) or severe (80%-100%) ischemia measured by a hydrogen gas clearance method[47]. There was no significant difference in AL in the animals with slight ischemia. In the groups with moderate ischemia, OP-41483 prevented the occurrence of AL, possibly by increasing the blood flow in the anastomotic area. All animals with severe bowel ischemia died due to major anastomotic dehiscence[47].
The enteral combination of neomycin and erythromycin decreased AL significantly in rats, whereas a parenteral combination of clindamycin and gentamicin was ineffective[48].
The extracellular matrix protein, amelogenin, enhanced anastomotic BPR. The mechanism of action remains elusive because amelogenin had no effect on hydroxyproline levels[49].
Vinpocetine[40], sildenafil[50] and the mast cell degranulating agent compound 48/80[51] had no statistically significant impact on BPR in ischemic colon.
All eight agents tested in rat I/R injury models were evaluated in single studies (Table 2). Before[52] or, more commonly, after construction of an anastomosis, I/R injury is induced by occluding mesenteric vessels of a 2-3 cm segment of the left colon[53] or by occluding the superior mesenteric artery[54-57] with microvascular clamps for 30-60 min before reperfusion.
| Ref. | Compound | Time of administration | Species | Sex | Sample size1 | Dosage | Route | Test | Test day | Effect2 |
| Tekin et al[56] | ATIII | Preoperative and postoperative | Rat | M | 16 | 2503 | iv | BPR | 6 | ↑ 74 |
| Unal et al[57] | Ethyl pyruvate | Preoperative | Rat | M | 24 | 504 | IP | BPR | 5 | ↑ 63 |
| Preoperative and postoperative | ↑ 69 | |||||||||
| Kabali et al[53] | NAC | Preoperative | Rat | F | 30 | 3004 | PO/IP | BPR | 7 | ↑ 25/↑ 37 |
| Aydin et al[55] | Tempol | Preoperative and postoperative | Rat | M | 20 | 304 | iv | BPR | 5 | ↑ 6 |
| Teke et al[58] | Activated protein C | Preoperative and postoperative | Rat | M | 24 | 0.14 | iv | BPR | 7 | ↑ 7 |
| Teke et al[59] | Caffeic acid phenethyl ester | Preoperative and postoperative | Rat | M | 24 | 0.00284 | iv + IP | BPR | 7 | ↑ 7 |
| Teke et al[54] | Pyrrolidine dithiocarbamate | Preoperative and postoperative | Rat | M | 20 | 1004 | iv | BPR | 6 | ↑ 6 |
| Celik et al[52] | Montelukast | Preoperative and postoperative | Rat | M | 24 | 104 | IP | BPR | 5 | ↑ 36 |
Notably, antithrombin III (ATIII)[56] and ethyl pyruvate[57] treatment increased anastomotic BPR in the I/R injured rats by more than 60%, possibly because of an increase in hydroxyproline concentrations.
The antioxidant N-acetyl-cysteine (NAC) significantly increased the hydroxyproline level; histological evaluation also revealed increased collagen deposition compared with the I/R injured control group, independent of the administration route[53].
Other compounds that prevented I/R-induced reductions in anastomotic patency included tempol[55], the immunomodulating compounds activated protein C[58], caffeic acid phenethyl ester[59] and pyrrolidine dithiocarbamate[54].
One study reported significantly increased anastomotic BPR and hydroxyproline levels after treatment with montelukast administered intraperitoneally[52].
Four different agents were investigated in models of colonic obstruction (Table 3). In these models, typically the left-sided colon was obstructed by suture ligation for 24 h. Re-laparotomy was then performed, the obstructed segment was excised, and an end-to-end anastomosis was constructed[23,60-63].
| Ref. | Compound | Time of administration | Species | Sex | Sample size1 | Dosage | Route | Test | Test day | Effect2 |
| Erbil et al[23] | PI | Preoperative | Rat | M | 144 | 103 | Lavage | BPR | 3/6 | ↑ 44/↑ 42 |
| L-NAME | 0.13 | ↓ 26/↓ 28 | ||||||||
| Aguilar-Nascimento et al[60] | PI | Preoperative | Rat | M | 40 | 103 | Lavage | BPR | 3/6 | NS/↑ 41 |
| BST | NS/↑ 28 | |||||||||
| Faruquzzaman et al[63] | EPO | Postoperative | Guinea pig | M | 20 | 5004 | SC | BPR | 7 | ↑ 15 |
| Moran et al[61] | EPO | Postoperative | Rat | M | 20 | 5004 | SC | BPR | 7 | ↑ 12 |
| Galanopoulos et al[62] | Iloprost | Postoperative | Rat | M | 40 | 0.0025 | IP | BPR | 4/8 | ↑ 115/↑ 74 |
| AL | NS/NS | |||||||||
| 6Gulcelik et al[17] | GM-CSF | Intraoperative | Rat | M | 44 | 0.0505 | LO | BPR | 3 | ↑ 24 |
In rats with an obstructed colon, intraoperative lavage with povidone iodine (PI) increased anastomotic BPR significantly on day 6 compared with untreated controls in two independent studies[23,60]. Because the BPR was similar to lavage with saline alone, the additional value of PI remains questionable[23,60]. NG-nitro-L-arginine methyl ester, an inhibitor of nitric oxide synthase, was found to be detrimental to anastomotic healing in rats with an obstructed colon[23]. Intra-abdominal irrigation is time consuming, costly, cumbersome and possibly increases the risk of spillage[64,65]. These circumstances should be taken into account when investigating new lavage agents.
EPO administered after construction of the anastomosis of the obstructed colon significantly increased BPR on day 7 in rats and guinea pigs[61,63]. EPO possibly enhanced anastomotic healing through increased neovascularization and fibroblast proliferation leading to more collagen in the anastomotic wound[61,63]. Therefore, further exploration of EPO to improve anastomotic wound healing under these conditions seems justified.
Iloprost increased BPR on days 4 and 8 in rats, possibly by stimulating angiogenesis and fibroblast activity[62]. Moreover, iloprost reduced the levels of immunodetectable MMP-13 in the anastomotic tissue, which may explain the increased collagen deposition with iloprost. Significantly more intra-abdominal adhesions formed, which were assessed according to the scale of van der Ham et al[66], in the rats with obstruction compared with the animals without obstruction[62]. The prostacycline analog iloprost did not reduce adhesion formation in the obstructed animals[63]. Although iloprost seemed to reduce AL (10% vs 30% for saline controls), this difference did not reach statistical significance[63].
Obstructive jaundice was modeled by ligation of the common bile duct. The anastomosis was constructed 7 d later[17]. GM-CSF, the only agent investigated in this clinical condition, increased BPR and the hydroxyproline level[17] (Table 3). Increased mononuclear infiltration of the anastomoses was suggested to be the mechanism for the improved anastomotic wound healing with GM-CSF[17].
Sixteen different compounds were identified, none of which qualified for the meta-analysis (Table 4). Experimental peritonitis is commonly established by puncture of the colon[67], ligated cecum[68-71], or intraperitoneal administration of fecal suspension[72], Escherichia coli suspension[73] or the Gram-negative wall component lipopolysaccharide (endotoxin)[74]. Anastomoses were then performed 5-14 h later. In one study, the cecum was ligated and punctured after the anastomosis was performed[75].
| Ref. | Compound | Time of administration | Species | Sex | Sample size1 | Dosage | Route | Test | Test day | Effect2 |
| Ayten et al[67] | Sildenafil | Postoperative | Rat | F | 14 | 83 | IP | BPR | 7 | ↑ 43 |
| Diller et al[74] | ATIII | Intraoperative | Mouse | M | 60 | 2504 | iv | BPR | 2 | ↑ 34 |
| 4 | NS | |||||||||
| 7 | ↑ 38 | |||||||||
| Gunerhan et al[72] | UFH | Postoperative | Rat | M | 45 | 503 | SC | BPR | 7 | ↑ 12 |
| LMWH | 1.53 | ↑ 19 | ||||||||
| Onur et al[76] | Ethyl pyruvate | Postoperative | Rat | N/A | 20 | 503 | IP | BPR | 7 | ↑ 51 |
| Aytekin et al[68] | Tempol | Preoperative and postoperative | Rat | M | 20 | 303 | iv | BPR | 6 | ↑ 10 |
| Ergin et al[75] | G-CSF | Preoperative and postoperative | Rat | M | 20 | 0.0503 | SC | BPR | 4 | ↑ 26 |
| Levamisole | 20 | 53 | PO | NS | ||||||
| Teke et al[69] | Activated protein C | Preoperative and postoperative | Rat | M | 24 | 0.13 | iv | BPR | 7 | ↑ 9 |
| Teke et al[70] | Caffeic acid phenethyl ester | Preoperative and postoperative | Rat | M | 24 | 0.00283 | IP | BPR | 7 | ↑ 9 |
| Teke et al[71] | Pyrrolidine dithiocarbamate | Preoperative and postoperative | Rat | M | 20 | 1003 | iv | BPR | 6 | ↑ 7 |
| Akkuş et al[73] | Taurolidine | Intraoperative | Rat | F | 40 | 0.55 | Lavage | BPR | 3/7 | ↑ 26/↑ 12 |
| Bicalho et al[78] | Chlorhexidine | Intraoperative | Rat | M | 16 | 0.055 | Lavage | BPR | 7 | NS |
| Wang et al[79] | Hydroxyethyl starch | Preoperative and postoperative | Rat | M | 32 | 7.56 | iv | BPR | 5 | NS |
| 156 | ↑ 9 | |||||||||
| 306 | ↓ 5 | |||||||||
| Wang et al[80] | Hydroxyethyl starch | Preoperative and postoperative | Rat | M | 20 | 156 | iv | BPR | 5 | ↑ 9 |
| Sucullu et al[81] | HBOT | Postoperative | Rat | M,F | 32 | BPR | 3/7 | ↑ 186/↑ 74 | ||
| Rocha et al[82] | HBOT | Postoperative | Rat | M | 30 | BST | 5 | NS | ||
| Vaneerdeweg et al[83] | Gentamicin | Intraoperative | Rat | M | 30 | 2.67/123 | LO/IM | BPR | 4 | NS/NS |
In one study, the vasomodulating agent sildenafil was administered intraperitoneally after the anastomoses were constructed in female rats with peritonitis. Sildenafil decreased intra-abdominal adhesions and increased BPR by 43% on day 7 compared with the controls that received saline alone[67]. Furthermore, sildenafil stimulated new vessel formation in the anastomoses[67]. In another rodent peritonitis model, intravenous administration of the thrombin inhibitor ATIII increased anastomotic BPR by 34% on day 2 and by 38% on day 7 compared with the peritonitis control[74]. Diller et al[74] attributed the improved anastomotic healing with ATIII to increased numbers of perfused capillaries and reduced clot formation, although these improvements failed to reach the levels of the non-infected controls.
Unfractionated heparin (UFH) and low molecular weight heparin (LMWH) prevented the reduction in BPR in animals with peritonitis[72]. Histopathological examinations revealed increased vascularization and collagen formation of the anastomotic wounds treated with UFH and LMWH[72]. Notably, UFH and LMWH facilitate intraperitoneal bacterial clearance by preventing formation of fibrin that may act as a reservoir for bacteria[72].
Ethyl pyruvate increased BPR by 51%, possibly due to its anti-inflammatory effects[76].
Reactive oxygen species (ROS) are thought to delay wound healing under septic conditions. Tempol is a stable piperidine nitroxide that may dampen the negative impact of ROS through its intracellular scavenging capacity. Tempol also restored glutathione levels and decreased the polymorphonuclear neutrophil counts in the anastomoses[68]. These effects possibly contributed to the elevated hydroxyproline and BPR levels with tempol[68].
The anti-inflammatory immunomodulating granulocyte-CSF (G-CSF)[75], activated protein C[69], caffeic acid phenethyl ester[70] and pyrrolidine dithiocarbamate[71] are attractive for prevention of the deleterious effects of peritonitis and also increase anastomotic BPR. Although G-CSF increased hydroxyproline levels, they were still lower than in the normal control group without peritonitis[75]. Activated protein C has been shown to reduce 28-d all-cause mortality in sepsis patients and is now approved for the treatment of patients with severe sepsis[69,77]. The usefulness of activated protein C in colonic anastomotic wound healing in the presence of peritonitis will require more study because of the increased risk of bleeding[77].
Abdominal lavage with the taurine derivative taurolidine increased BPR on days 3 and 7[73]. Chlorhexidine lavage had no significant effect on BPR compared with 4-time lavage with 5 mL of sterile saline prior to construction of the anastomosis[78].
Intravenous administration of hydroxylethyl starch (HES) at 15 mL/kg increased BPR on postoperative day 5 in two studies carried out by the same research group[79,80], whereas 30 mL/kg was detrimental to anastomotic healing[80]. These findings may be explained by the anti-inflammatory effects of HES at 15 mL/kg[79,80], whereas at higher doses, HES reduces platelet aggregation to the injured endothelium[80]. These facts make HES less practical for use in a clinical setting.
Postoperative HBOT in rats increased BPR by 186% on day 3 and by 74% on day 7[81]. In another study, HBOT had no effect on BST on day 5[82].
Local or systemic application of gentamicin[83], as well as levamisole[75], had no effect on BPR in male rats with peritonitis.
Five different compounds were tested in rats treated with different chemotherapeutic agents (Table 5). In these studies, 5-fluorouracil was given preoperatively[84,85] or on postoperative days 3-8[86-90]. Mitomycin-C was given as a single intraoperative dose[91].
| Ref. | Compound | Time of administration | Species | Sex | Sample size1 | Dosage | Route | Test | Test day | Effect2 |
| Cetinkaya et al[91] | GM-CSF | Postoperative | Rat | N/A | 54 | 0.0503 | LO | BPR | 3 | ↑ 26 |
| Erdem et al[86] | GM-CSF | Postoperative | Rat | N/A | 30 | 0.0503 | LO | BPR | 3 | ↑ 98 |
| de Waard et al[87] | GM-CSF | Postoperative | Rat | M | 31 | 0.0053 | IP | BPR/BST | 7 | ↓ 35/NS |
| Interleukin-2 | 42 × 106 | SC | NS/NS | |||||||
| Bostanoğlu et al[88] | Iloprost | Postoperative | Rat | M | 38 | 0.0023 | N/A | BPR | 3/7 | ↑ 63/NS |
| Vasiliadis et al[90] | Iloprost | Postoperative | Rat | F | 34 | 0.0023 | IP | BPR | 5/8 | ↑ 44/NS |
| AL | ↓ 30/↓ 30 | |||||||||
| Zacharakis et al[89] | IGF-1 | Postoperative | Rat | M | 32 | 23 | IP | BPR/AL | 7 | ↑ 53/NS |
| Erenoğlu et al[84] | HBOT | Postoperative | Rat | M | 20 | BPR | 7 | ↑ 26 | ||
| 5Yildiz et al[85] | HBOT | Postoperative | Rat | F | 24 | BPR | 5 | NS |
Three studies investigated the effect of GM-CSF[86,87,91] and were subjected to meta-analysis. The combined estimate demonstrated that GM-CSF failed to increase anastomotic BPR (95%CI: -20 to 21 mmHg, P = 0.97) compared with controls (Figure 3B). The inconsistency between studies was large (I2 = 92%). The two studies demonstrating improved anastomotic healing also reported a significantly increased hydroxyproline concentration in the anastomoses, as well as distinct histological changes, including increased mononuclear infiltration compared with chemotherapy alone (fluorouracil or mitomycin-C)[86,91]. de Waard et al[87] found that GM-CSF increased BPR, but not BST, in fluorouracil-treated rats. They also applied a considerably lower dose of GM-CSF (5 μg)[87] than the other two research groups (50 μg)[86,91]. In addition, GM-CSF was administered intraperitoneally and not locally. Taken together, these data indicate that the GM-CSF dose used by de Waard et al[87] was too low. In contrast, a single local application of GM-CSF in expanded polytetrafluoroethylene tubes implanted subcutaneously in humans inhibited collagen deposition dose-dependently and resulted in systemic effects on wound healing at doses of 4 μg or more[92].
Iloprost enhanced BPR anastomotic healing on postoperative day 3[88] and day 5[89] compared with both the chemotherapeutic group and the non-chemotherapeutic group, but not at later time points[88,89]. Interestingly, iloprost significantly decreased the rate of AL from 30% to 0% in rats receiving chemotherapy[90]. The positive effects on anastomotic strength in models of chemotherapy were possibly due to increased angiogenesis[90] and collagen deposition with iloprost[88,90]. Furthermore, iloprost significantly reduced the severity of intra-abdominal adhesions compared with the chemotherapeutic control group[90].
A single study on IGF-1 treatment reported normalization of anastomotic BPR and hydroxyproline levels on day 7 in fluorouracil-treated rats[89]. IGF-1 had no significant effects on AL[89].
Postoperative HBOT increased BPR on day 7 in one study[84] but had no significant effect on day 5 in another study, in which the rats received combined chemo- and radiotherapy before surgery[85].
Interleukin-2 administered postoperatively had no effect on either BPR or BST[87].
None of the eight agents identified and investigated in radiotherapy models qualified for meta-analysis (Table 6). Animals were irradiated with half-body radiation[27,93] or abdomino-pelvic radiation[94-96] in a single dose and as rectosigmoid radiation[97] 5 d a week for 40-45 d.
| Ref. | Compound | Time of administration | Species | Sex | Sample size1 | Dosage | Route | Test | Test day | Effect2 |
| Demir et al[96] | NAC | Preoperative and postoperative | Rat | M | 24 | 3005 | PO/IP | BPR | 4 | ↑ 126/↑ 182 |
| Ozdemir et al[94] | Amifostine | Preoperative | Rat | F | 20 | 2005 | IP | BPR | 5 | ↑ 16 |
| Carroll et al[93] | Ribose-cysteine3 | Preoperative | Rat | M | 72 | 20005 | IP | BPR | 7 | ↑ 50 |
| Ribose-cysteine4 | Preoperative | 50005 | IP | N/A | ||||||
| Ribose-cysteine3 + glutamine3 | Preoperative and postoperative | 20005 + 36 | IP + PO | NS | ||||||
| Amifostine3 | Preoperative | 2505 | IP | ↑ 46 | ||||||
| Amifostine4 | Preoperative | 2505 | IP | NS | ||||||
| MgCl23 + ATP3 | Preoperative | 107 + 507 | iv | ↑ 67 | ||||||
| Rowe et al[97] | Ribose-cysteine | Preoperative | Pig | M | 12 | 10005 | iv | BPR | 9/11 | NS |
| Değer et al[27] | Pycnogenol | Preoperative | Rat | M | 40 | 2005 | PO | BPR | 3/7 | ↑ 19/↑ 38 |
| Ozel Turkcu et al[95] | EPO | Preoperative and postoperative | Rat | M | 16 | 5008 | IM | BPR | N/A | NS |
NAC is theoretically attractive in preventing oxidative damage after radiotherapy. NAC administered before and after construction of the anastomosis also increased BPR[96]. In one study, NAC treatment increased the levels of superoxide dismutase and glutathione, but decreased malondialdehyde[96]. Superoxide dismutase and glutathione are known to neutralize toxic substances in the cell, whereas malondialdehyde is a marker for oxidative stress[96].
Other compounds found beneficial for the prevention of the deleterious effects of preoperative radiation include amifostine[93,94] and magnesium chloride in combination with adenosine triphosphate[93].
Ribose-cysteine[93] administered before surgery improved anastomotic healing day 7 in rats receiving abdominal radiation with 40 GY, but not in irradiated pig colon days 9-11[97]. No beneficial effects were reported by Carroll et al[93], who investigated the effects of ribose-cysteine combined with glutamine.
Pycnogenol administered preoperatively increased BPR on postoperative days 3 and 7 in male rats[27].
EPO had no significant effect on anastomotic strength[95].
We have previously reviewed therapeutics intended to enhance normal anastomotic repair in the colorectal region[28]. In this paper we systematically retrieved publications on therapeutic agents intended to promote anastomotic wound healing under the influence of complicating factors, including ischemia, I/R injury, colonic obstruction, obstructive jaundice, peritonitis, chemotherapy and radiotherapy. The majority of the 48 different therapeutic compounds identified were only assessed in one study and/or in one complicated model. Meta-analysis was performed for HBOT and GM-CSF. Postoperative HBOT significantly improved wound healing in a rat model complicated by ischemia in the anastomosis. GM-CSF failed to show a beneficial effect on anastomotic healing in conjunction with chemotherapy. On the other hand, positive effects of GM-CSF were found in models of segmental ischemia[46] and obstructive jaundice[17]; these findings make this agent interesting for further investigation. Iloprost was found to be beneficial for early healing of anastomotic wounds in rats with colonic obstruction[62] and in rats exposed to chemotherapy[88,90], calling for further studies with this agent. The positive actions of NAC after I/R injury[53] and radiotherapy[96] justify further investigations on this antioxidant, as well.
Because the 65 pre-clinical studies examined in our review used surrogate outcomes in models mimicking clinical phenomena, the results do not directly translate into clinical AL. Only 12 (25%) of the agents were investigated more than once in the same model. Furthermore, 13 (27%) therapeutic compounds were tested in two or more models of complicated anastomotic wound healing. We were unable to assess publication bias, for example, with Funnel plots, due to the small sample sizes of the included studies[98].
Further exploration of the therapeutic agents identified in this review may be the next step to reach more robust conclusions regarding whether the agents could be effective in preventing AL in high-risk patients.
Despite improvements in preoperative management and surgical techniques, anastomotic leakage remains a major complication in gastrointestinal surgery. There are no therapeutic agents with documented prophylactic effect against this serious postoperative surgical complication. A recently published meta-analysis identified seven compounds with the potential to improve anastomotic healing under non-complicated conditions.
This study is the first to systematically review therapeutic agents that are potentially capable of abolishing or reducing the deleterious effects of various known complicating factors on anastomotic healing.
The search identified 48 different therapeutic agents. A meta-analysis indicated that hyperbaric oxygen therapy improved colonic anastomotic repair in models of ischemia.
This systematic review identified therapeutic substances from pre-clinical studies on complicated anastomotic wound healing that would be worthwhile exploring further for the prevention of anastomotic leakage.
Anastomotic leakage results in contamination of the abdominal cavity with intestinal contents, leading to peritonitis or sepsis, or in worst case mortality.
This systematic review assessed the efficacy of therapeutic agents against anastomotic leakage in animal models. The manuscript is excellent, very well written and educational despite being research-oriented. A widespread prospective multicenter trial would be ideal to follow up on the collected information.
P- Reviewer: Bandyopadhyay S, Mizuguchi T, Venskutonis D S- Editor: Kong JX L- Editor: A E- Editor: Liu SQ
| 1. | Krarup PM, Jorgensen LN, Andreasen AH, Harling H. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis. 2012;14:e661-e667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 3. | Pommergaard HC, Gessler B, Burcharth J, Angenete E, Haglind E, Rosenberg J. Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2014;16:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006;49:1719-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, Rutten HJ, van de Velde CJ. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 508] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 7. | Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis. 2008;10:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 591] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 9. | Krarup PM, Eld M, Heinemeier K, Jorgensen LN, Hansen MB, Ågren MS. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int J Colorectal Dis. 2013;28:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ågren MS, Andersen TL, Mirastschijski U, Syk I, Schiødt CB, Surve V, Lindebjerg J, Delaissé JM. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006;140:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Schrock TR, Deveney CW, Dunphy JE. Factor contributing to leakage of colonic anastomoses. Ann Surg. 1973;177:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 355] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Sheridan WG, Lowndes RH, Young HL. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;30:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Kuzu MA, Köksoy C, Kale IT, Tanik A, Terzi C, Elhan AH. Reperfusion injury delays healing of intestinal anastomosis in a rat. Am J Surg. 1998;176:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kologlu M, Yorganci K, Renda N, Sayek I. Effect of local and remote ischemia-reperfusion injury on healing of colonic anastomoses. Surgery. 2000;128:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Kuzu MA, Tanik A, Kale IT, Aşlar AK, Köksoy C, Terzi C. Effect of ischemia/reperfusion as a systemic phenomenon on anastomotic healing in the left colon. World J Surg. 2000;24:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Golub R, Golub RW, Cantu R, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997;184:364-372. [PubMed] |
| 17. | Gulcelik MA, Dinc S, Bir F, Elitok O, Alagol H, Oz M. Locally applied molgramostim improves wound healing at colonic anastomoses in rats after ligation of the common bile duct. Can J Surg. 2005;48:213-218. [PubMed] |
| 18. | Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. 2002;26:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Morgenstern L, Yamakawa T, Ben-Shoshan M, Lippman H. Anastomotic leakage after low colonic anastomosis. Clinical and experimental aspects. Am J Surg. 1972;123:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Lee WS, Yun SH, Roh YN, Yun HR, Lee WY, Cho YB, Chun HK. Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg. 2008;32:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Adas G, Percem A, Adas M, Kemik O, Arikan S, Ustek D, Cakiris A, Abaci N, Kemik AS, Kamali G. VEGF-A and FGF gene therapy accelerate healing of ischemic colonic anastomoses (experimental study). Int J Surg. 2011;9:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Garcia JG, Criado FJ, Persona MA, Alonso AG. Healing of colonic ischemic anastomoses in the rat: role of superoxide radicals. Dis Colon Rectum. 1998;41:892-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Erbil Y, Calis A, Berber E, Mercan S. The effect of intraoperative colonic lavage with NG-nitro-L-arginine methyl ester (L-NAME) on anastomotic healing in the presence of left-sided colonic obstruction in the rat. Surg Today. 2000;30:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Phillips RK, Hittinger R, Fry JS, Fielding LP. Malignant large bowel obstruction. Br J Surg. 1985;72:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 270] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Ahrendt GM, Tantry US, Barbul A. Intra-abdominal sepsis impairs colonic reparative collagen synthesis. Am J Surg. 1996;171:102-107; discussion 107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Martens MF, Hendriks T, Wobbes T, De Pont JJ. Intraperitoneal cytostatics impair early post-operative collagen synthesis in experimental intestinal anastomosesP6. Br J Cancer. 1992;65:649-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Değer KC, Şeker A, Özer I, Bostancı EB, Dalgıç T, Akmansu M, Ekinci Ö, Erçin U, Bilgihan A, Akoğlu M. The effects of Pycnogenol(®) on colon anastomotic healing in rats given preoperative irradiation. Int J Surg. 2013;11:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Øines MN, Krarup PM, Jorgensen LN, Ågren MS. Pharmacological interventions for improved colonic anastomotic healing: a meta-analysis. World J Gastroenterol. 2014;20:12637-12648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Yauw ST, Wever KE, Hoesseini A, Ritskes-Hoitinga M, van Goor H. Systematic review of experimental studies on intestinal anastomosis. Br J Surg. 2015;102:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8058] [Article Influence: 537.2] [Reference Citation Analysis (2)] |
| 31. | Hamzaoğlu I, Karahasanoğlu T, Aydin S, Sahin DA, Carkman S, Sariyar M, Alemdaroğlu K. The effects of hyperbaric oxygen on normal and ischemic colon anastomoses. Am J Surg. 1998;176:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Yarimkaya A, Apaydin B, Unal E, Karabicak I, Aydogan F, Uslu E, Erginoz E, Artis T, Eyuboglu E. Effects of recombinant human growth hormone and nandrolone phenylpropionate on the healing of ischemic colon anastomosis in rats. Dis Colon Rectum. 2003;46:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Yagci G, Ozturk E, Ozgurtas T, Gorgulu S, Kutlu OC, Topal T, Cetiner S, Tufan T. Preoperative and postoperative administration of hyperbaric oxygen improves biochemical and mechanical parameters on ischemic and normal colonic anastomoses. J Invest Surg. 2006;19:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Parra-Membrives P, Ruiz-Luque V, Escudero-Severín C, Aguilar-Luque J, Méndez-García V. Effect of pentoxifylline on the healing of ischemic colorectal anastomoses. Dis Colon Rectum. 2007;50:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Guzel S, Sunamak O, AS A, Celik V, Ferahman M, Nuri MM, Gazioglu E, Atukeren P, Mutlu O. Effects of hyperbaric oxygen and Pgg-glucan on ischemic colon anastomosis. World J Gastroenterol. 2006;12:1421-1425. [PubMed] |
| 36. | Kemik O, Adas G, Arikan S, Gurluler E, Dogan Y, Toklu AS, Kapran Y, Kuntsal L, Purisa S, Kemik A. Evaluation of the effects of hyperbaric oxygen treatment and enoxaparin on left colon anastomosis. An experimental study. Eur Rev Med Pharmacol Sci. 2013;17:2286-2292. [PubMed] |
| 37. | Rijcken E, Sachs L, Fuchs T, Spiegel HU, Neumann PA. Growth factors and gastrointestinal anastomotic healing. J Surg Res. 2014;187:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Saribeyoğlu K, Baca B, Hamzaoğlu I, Pekmezci S, Karahasanoğlu T, Hamzaoğlu H. Does becaplermin (platelet-derived growth factor-BB) reverse detrimental effects of ischemia on colonic anastomosis? Dis Colon Rectum. 2003;46:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Tasdelen A, Algin C, Ates E, Kiper H, Inal M, Sahin F. Effect of leptin on healing of colonic anastomoses in rats. Hepatogastroenterology. 2004;51:994-997. [PubMed] |
| 40. | Sümer A, Altınlı E, Senger S, Köksal N, Onur E, Eroğlu E, Güneş P. Effect of pentoxifylline and vinpocetine on the healing of ischemic colon anastomosis: an experimental study. Ulus Travma Acil Cerrahi Derg. 2011;17:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Karatepe O, Kurtulus I, Yalcin O, Battal M, Kamali G, Aydin T. Adrenomedulline improves ischemic left colonic anastomotic healing in an experimental rodent model. Clinics (Sao Paulo). 2011;66:1805-1810. [PubMed] |
| 42. | Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. The clinical relevance of adrenomedullin: a promising profile? Pharmacol Ther. 2004;103:179-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Cetinkaya Z, Esen K, Ozercan IH, Ustundag B, Ayten R, Aygen E. The effect of Bosentan on healing of colonic anastomosis. World J Emerg Surg. 2006;1:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Adas G, Arikan S, Karatepe O, Kemik O, Ayhan S, Karaoz E, Kamali G, Eryasar B, Ustek D. Mesenchymal stem cells improve the healing of ischemic colonic anastomoses (experimental study). Langenbecks Arch Surg. 2011;396:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Adas G, Kemik O, Eryasar B, Okcu A, Adas M, Arikan S, Erman G, Kemik AS, Kamali G, Dogan Y. Treatment of ischemic colonic anastomoses with systemic transplanted bone marrow derived mesenchymal stem cells. Eur Rev Med Pharmacol Sci. 2013;17:2275-2285. [PubMed] |
| 46. | Dinc S, Gulcelik MA, Kuru B, Ergeneci D, Camlibel M, Caydere M, Alagol H. Effects of locally applied recombinant human granulocyte-macrophage colony-stimulating factor on ischemic bowel anastomoses in rat. Eur Surg Res. 2004;36:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Ikeda S, Itoh H, Oohata Y, Nakayama F. Effect of a new prostacyclin analogue on anastomosis of ischemic colon in dogs. Dis Colon Rectum. 1988;31:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Cohen SR, Cornell CN, Collins MH, Sell JE, Blanc WA, Altman RP. Healing of ischemic colonic anastomoses in the rat: role of antibiotic preparation. Surgery. 1985;97:443-446. [PubMed] |
| 49. | Karataş A, Kocael A, Aytaç E, Gökcal F, Salihoğlu Z, Uzun H, Paksoy M. Amelogenin (an extracellular matrix protein) application on ischemic colon anastomosis in rats. Ulus Travma Acil Cerrahi Derg. 2010;16:487-490. [PubMed] |
| 50. | Irkorucu O, Ucan BH, Cakmak GK, Emre AU, Tascilar O, Ofluoglu E, Bahadir B, Karakaya K, Demirtas C, Ankarali H. Does sildenafil reverse the adverse effects of ischemia on ischemic colon anastomosis: yes, ‘no’. Int J Surg. 2009;7:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Coneely J, Kennelly R, Bouchier-Hayes D, Winter DC. Mast cell degranulation is essential for anastomotic healing in well perfused and poorly perfused rat colon. J Surg Res. 2010;164:e73-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Celik A, Ergun E, Koksal N, Celik AS, Altinli E, Uzun MA, Eroglu E, Kemik A. Effects of montelukast on the healing of ischemic colon anastomoses. Am J Surg. 2013;206:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Kabali B, Girgin S, Gedik E, Ozturk H, Kale E, Buyukbayram H. N-acetylcysteine prevents deleterious effects of ischemia/reperfusion injury on healing of colonic anastomosis in rats. Eur Surg Res. 2009;43:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Teke Z, Aytekin FO, Kabay B, Yenisey C, Aydin C, Tekin K, Sacar M, Ozden A. Pyrrolidine dithiocarbamate prevents deleterious effects of remote ischemia/reperfusion injury on healing of colonic anastomoses in rats. World J Surg. 2007;31:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Aydin C, Teke Z, Aytekin F, Yenisey C, Kabay B, Simsek NG, Tekin K. Tempol prevents harmful effects of remote ischemia reperfusion injury on healing of experimental colonic anastomoses. Int J Colorectal Dis. 2007;22:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Tekin K, Aytekin F, Ozden A, Bilgihan A, Erdem E, Sungurtekin U, Güney Y. Antithrombin III prevents deleterious effects of remote ischemia-reperfusion injury on healing of colonic anastomoses. Am J Surg. 2002;184:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Unal B, Karabeyoglu M, Huner T, Canbay E, Eroglu A, Yildirim O, Dolapci M, Bilgihan A, Cengiz O. Ethyl pyruvate protects colonic anastomosis from ischemia-reperfusion injury. Surg Innov. 2009;16:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Teke Z, Sacar M, Yenisey C, Atalay AO, Bicakci T, Erdem E. Activated protein C prevents deleterious effects of remote reperfusion injury caused by intestinal ischemia on wound healing in the left colonic anastomoses: an experimental study in the murine model. Am J Surg. 2008;196:774-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Teke Z, Bostanci EB, Yenisey C, Kelten EC, Sacar M, Simsek NG, Duzcan SE, Akoglu M. Caffeic acid phenethyl ester prevents detrimental effects of remote ischemia-reperfusion injury on healing of colonic anastomoses. J Invest Surg. 2013;26:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Aguilar-Nascimento JE, Mathie RT, Man WK, Williamson RC. Enhanced intra-anastomotic healing by operative lavage with nutrient solutions in experimental left-sided colonic obstruction. Br J Surg. 1995;82:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Moran M, Ozmen MM, Duzgun AP, Gok R, Renda N, Seckin S, Coskun F. The effect of erythropoietin on healing of obstructive vs nonobstructive left colonic anastomosis: an experimental study. World J Emerg Surg. 2007;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Galanopoulos G, Raptis D, Pramateftakis MG, Mantzoros I, Kanellos I, Lazarides C. The effects of iloprost on colonic anastomotic healing in rats under obstructive ileus conditions. J Surg Res. 2014;189:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Faruquzzaman SK. The healing role of erythropoietin in the obstructive vs nonobstructive left colonic anastomosis. Bratisl Lek Listy. 2009;110:530-535. [PubMed] |
| 64. | Naraynsingh V, Rampaul R, Maharaj D, Kuruvilla T, Ramcharan K, Pouchet B. Prospective study of primary anastomosis without colonic lavage for patients with an obstructed left colon. Br J Surg. 1999;86:1341-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Biondo S, Parés D, Kreisler E, Ragué JM, Fraccalvieri D, Ruiz AG, Jaurrieta E. Anastomotic dehiscence after resection and primary anastomosis in left-sided colonic emergencies. Dis Colon Rectum. 2005;48:2272-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | van der Ham AC, Kort WJ, Weijma IM, van den Ingh HF, Jeekel J. Effect of fibrin sealant on the healing colonic anastomosis in the rat. Br J Surg. 1991;78:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Ayten R, Cetinkaya Z, Girgin M, Ozercan I, Ustundag B, Aygen E. The effects of intraperitoneal sildenafil administration on healing of left colonic anastomoses and intra-abdominal adhesion formation in the presence of intra-abdominal infection. Dis Colon Rectum. 2008;51:1837-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Aytekin FO, Teke Z, Aydin C, Kabay B, Yenisey C, Sacar S, Demir EM, Tekin K. Effects of a membrane-permeable radical scavenger, Tempol, on healing of colonic anastomoses in the cecal ligation and puncture model of polymicrobial sepsis in rats. Am J Surg. 2007;193:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Teke Z, Sacar S, Yenisey C, Atalay AO, Kavak T, Erdem E. Role of activated protein C on wound healing process in left colonic anastomoses in the presence of intra-abdominal sepsis induced by cecal ligation and puncture: an experimental study in the rat. World J Surg. 2008;32:2434-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Teke Z, Bostanci EB, Yenisey C, Kelten EC, Sacar S, Simsek NG, Duzcan SE, Akoglu M. Effects of caffeic acid phenethyl ester on anastomotic healing in secondary peritonitis. J Invest Surg. 2012;25:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Teke Z, Aytekin FO, Aydin C, Kabay B, Yenisey C, Sacar S, Simsek NG, Tekin K. Effects of pyrrolidine dithiocarbamate on healing of colonic anastomoses in the cecal ligation and puncture model of intraperitoneal sepsis in rats. World J Surg. 2007;31:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Gunerhan Y, Koksal N, Gul O, Uzun MA, Guneş P, Adaleti R. Effects of unfractionated heparin and low-molecular-weight heparin on colonic anastomoses in the presence of experimental peritonitis. Eur Surg Res. 2006;38:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Akkuş A, Gülmen M, Cevik A, Bildik N, Sad O, Oztürk E, Barişik NO. Effect of peritoneal lavage with taurolidine on primary colonic anastomosis in a rat model of secondary peritonitis. Surg Today. 2006;36:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Diller R, Stratmann U, Minin E, von Eiff C, Bäumer G, Huismans H, Helmschmied T, Becker K, Spiegel HU. ATIII attenuates endotoxemia induced healing impairment in the colon. J Surg Res. 2009;157:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Ergin E, Paksoy M, Erguney S, Uzun H, Sakoglu N. The effects of the immunomodulators on the colonic anastomosis in an experimental model of intraperitoneal sepsis. Hepatogastroenterology. 2004;51:439-442. [PubMed] |
| 76. | Onur E, Akalin B, Memisoglu K, Karip AB, Aydin MT, Altun H, Ekci B. Ethyl pyruvate improves healing of colonic anastomosis in a rat model of peritonitis. Surg Innov. 2012;19:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4217] [Cited by in RCA: 3824] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 78. | Bicalho PR, Mayrink CA, Fernandes F, Alvarenga DG, Araujo ID, Nunes TA, Reis FA. Treatment with chlorhexidine modifies the healing of colon anastomosis in rats. J Invest Surg. 2011;24:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Wang P, Gong G, Li Y, Li J. Hydroxyethyl starch 130/0.4 augments healing of colonic anastomosis in a rat model of peritonitis. Am J Surg. 2010;199:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Wang P, Li Y, Li J. Influence of hydroxyethyl starch on healing of colonic anastomosis in a rat model of peritonitis. J Invest Surg. 2009;22:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Sucullu I, Sinan H, Filiz AI, Yildiz S, Yucel E, Kurt Y, Akin ML. The effects of hyperbaric oxygen therapy on colonic anastomosis in rats with peritonitis. J Invest Surg. 2008;21:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Rocha AA, Leal RF, Ayrizono Mde L, Chung WF, Coy CS, Lee HD, Fagundes JJ. Hyperbaric oxygen therapy and mechanical resistence of the colonics anastomosis in rats with peritonitis. Acta Cir Bras. 2010;25:368-374. [PubMed] |
| 83. | Vaneerdeweg W, Hendriks JM, Lauwers PR, Ieven M, Eyskens EJ. Effect of gentamicin-containing sponges on the healing of colonic anastomoses in a rat model of peritonitis. Eur J Surg. 2000;166:959-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Erenoğlu C, Uluutku H, Emeksiz S, Akin ML, Foley E, Celenk T. Effect of hyperbaric oxygen on anastomoses created under the influence of 5-FU. Undersea Hyperb Med. 2003;30:321-326. [PubMed] |
| 85. | Yildiz R, Can MF, Yagci G, Ozgurtas T, Guden M, Gamsizkan M, Ozturk E, Cetiner S. The effects of hyperbaric oxygen therapy on experimental colon anastomosis after preoperative chemoradiotherapy. Int Surg. 2013;98:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Erdem E, Dinç S, Erdem D, Ustün H, Caydere M, Alagöl H. Effects of intraperitoneal chemotherapy and GM-CSF on anastomotic healing: an experimental study in rats. J Surg Res. 2002;108:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | de Waard JW, Wobbes T, van der Linden CJ, Hendriks T. Retinol may promote fluorouracil-suppressed healing of experimental intestinal anastomoses. Arch Surg. 1995;130:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Bostanoğlu S, Dinçer S, Keskin A, Bostanoğlu A, Dursun A, Serim C. Beneficial effect of Iloprost on impaired colonic anastomotic healing induced by intraperitoneal 5-fluorouracil infusion. Dis Colon Rectum. 1998;41:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Zacharakis E, Demetriades H, Pramateftakis MG, Lambrou I, Zacharakis E, Zaraboukas T, Koliakos G, Kanellos I, Betsis D. Effect of IGF-I on healing of colonic anastomoses in rats under 5-FU treatment. J Surg Res. 2008;144:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Vasiliadis K, Pramateftakis MG, Blouhos K, Mantzoros I, Koliakos G, Zaraboukas T, Kanellos I, Demetriades H, Alamdari DH, Betsis D. Effect of iloprost on impaired anastomotic healing caused by 5-fluorouracil plus leucovorin. Dis Colon Rectum. 2007;50:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Cetinkaya K, Dinc S, Gulcelik MA, Renda N, Ustun H, Caydere M, Alagol H. Granulocyte macrophage-colony stimulating factor improves impaired anastomotic wound healing in rats treated with intraperitoneal mitomycin-C. Surg Today. 2005;35:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Jorgensen LN, Ågren MS, Madsen SM, Kallehave F, Vossoughi F, Rasmussen A, Gottrup F. Dose-dependent impairment of collagen deposition by topical granulocyte-macrophage colony-stimulating factor in human experimental wounds. Ann Surg. 2002;236:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Carroll MP, Zera RT, Roberts JC, Schlafmann SE, Feeney DA, Johnston GR, West MA, Bubrick MP. Efficacy of radioprotective agents in preventing small and large bowel radiation injury. Dis Colon Rectum. 1995;38:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Ozdemir CS, Burgazli KM, Beken-Ozdemir E, Akdere H, Mericliler M, Ozcelik MF. The effect of Amifostine (Ethyol) on intestinal anastomosis in rats with radiation enteritis. Eur Rev Med Pharmacol Sci. 2013;17:1351-1359. [PubMed] |
| 95. | Ozel Turkcu U, Cakmak GK, Demir EO, Bakkal H, Oner MO, Okyay RD, Bassorgun IC, Ciftcioglu MA. The effect of erythropoietin on anastomotic healing of irradiated rats. J Invest Surg. 2012;25:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Demir EO, Cakmak GK, Bakkal H, Turkcu UO, Kandemir N, Demir AS, Tascılar O. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J Invest Surg. 2011;24:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Rowe JK, Zera RT, Madoff RD, Fink AS, Roberts JC, Johnston GR, Feeney DA, Young HL, Bubrick MP. Protective effect of RibCys following high-dose irradiation of the rectosigmoid. Dis Colon Rectum. 1993;36:681-688. [PubMed] |
| 98. | Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |