Published online Apr 27, 2016. doi: 10.4240/wjgs.v8.i4.335
Peer-review started: May 8, 2015
First decision: August 4, 2015
Revised: November 24, 2015
Accepted: February 23, 2016
Article in press: February 24, 2016
Published online: April 27, 2016
Processing time: 353 Days and 20.1 Hours
AIM: To study the etiopathogenesis, management and outcome of duodenal injury post laparoscopic cholecystectomy (LC).
METHODS: A Medline search was carried out for all articles in English, on duodenal injury post LC, using the search word duodenal injury and LC. The cross references in these articles were further searched, for potential articles on duodenal injury, which when found was studied. Inclusion criteria included, case reports, case series, and reviews. Articles even with lack of details with some of the parameters studied, were also analyzed. The study period included all the cases published till January 2015. The data extracted were demographic details, the nature and day of presentation, potential cause for duodenal injury, site of duodenal injury, investigations, management and outcome. The model (fixed or random effect) for meta analyses was selected, based on Q and I2 statistics. STATA software was used to draw the forest plot and to compute the overall estimate and the 95%CI for the time of detection of injury and its outcome on mortality. The association between time of detection of injury and mortality was estimated using χ2 test with Yate’s correction. Based on Kaplan Meier survival curve concept, the cumulative survival probabilities at various days of injury was estimated.
RESULTS: Literature review detected 74 cases of duodenal injury, post LC. The mean age of the patients was 58 years (23-80 years) with 46% of them being males. The cause of injury was due to cautery (46%), dissection (39%) and due to retraction (14%). The injury was noted on table in 46% of the cases. The common site of injury was to the 2nd part of the duodenum with 46% above the papilla and 15% below papilla and in 31% to the 1st part of duodenum. Duodenorapphy (primary closure) was the predominant surgical intervention in 63% with 21% of these being carried out laparoscopically. Other procedures included, percutaneous drainage, tube duodenostomy, gastric resection, Whipple resection and pyloric exclusion. The day of detection among those who survived was a mean of 1.6 d (including those detected on table), compared to 4.25 d in those who died. Based on the random effect model, the overall mean duration of detection of injury was 1.6 (1.0-2.2) d (95%CI). Based on the fixed effect model, the overall mortality rate from these studies was 10% (0%-25%). On application of the Kaplan Meier survival probabilities, the cumulative probability of survival was 94%, if the injury was detected on day 1 and 80% if detected on day 2. In those that were detected later, the survival probabilities dropped steeply.
CONCLUSION: Duodenal injuries are caused by thermal burns or by dissection during LC and require prompt treatment. Delay in repair could negatively influence the outcome.
Core tip: Inadvertent duodenal injury is a rare potentially fatal complication of laparoscopic cholecystectomy. Such injuries often go unrecognized at the time of the procedure and manifest later with significant morbidity and mortality. Literature review revealed 74 cases of duodenal injury. The injury was caused by cautery in 46%, dissection in 39% and retraction in 14% of the cases. The predominant site of injury was to the 2nd part in 61% and in 31% to 1st part. Duodenorapphy was the primary treatment carried out in 63% of the cases among which 21% was laparoscopically. When detected on table, 88.9% survived in contrast to 76.5% detected later. Overall mortality was 18%. The major impact of this review in clinical practice is in emphasizing the need for prompt detection of a potential duodenal injury in every patient who has unexplained postoperative course following a difficult laparoscopic cholecystectomy due to gall bladder adhesions or dissection. The change of clinical practice it should lead to is an attempt by surgeons in early detection of potential duodenal injury in such patients, which could be achieved by estimating the amylase content in subhepatic fluid collection or by upper gastrointestinal contrast studies. It also highlights the need for immediate surgical repair as any delay beyond the first postoperative day has adverse effect on outcome.
- Citation: Machado NO. Duodenal injury post laparoscopic cholecystectomy: Incidence, mechanism, management and outcome. World J Gastrointest Surg 2016; 8(4): 335-344
- URL: https://www.wjgnet.com/1948-9366/full/v8/i4/335.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i4.335
Laparoscopic cholecystectomy (LC) is gold standard in the management of benign gall bladder disease[1]. Unfortunately, in a small percentage of cases, it is associated with serious complications, which could be life threatening[2-6]. These include those patients who sustain vascular or bowel injury[2,4,6]. Among the bowel injuries, duodenal injury is extremely rare complication of LC, with a outcome that is potentially fatal[4-26]. It is commonly unrecognized at the time of the procedure and is unfortunately diagnosed later when sepsis, peritonitis, intraperitoneal abscess or enterocutaneous fistula sets in[2,27]. Several factors may play a role in causing these injuries, including complexity of the case and the experience of the surgeon[5,6,15]. The incidence, mechanism of injury, diagnosis, management and outcome is described, along with the review of literature.
PubMed, EBSCO were searched for articles on duodenal injury during laparoscopic cholecystectomy.
The study included articles in English literature on duodenal injury post laparoscopic cholecystectomy. The articles included case reports and case series. The references in each of these articles were further studied for additional articles on duodenal injury, post laparoscopic cholecystectomy.
The key words used as search terms were “duodenal injury”, “laparoscopic cholecystectomy”, “laparoscopic complications”.
Various details were extracted from these articles. The variables studied included demographic details, presentation of symptoms and signs, day of detection of injury, investigations used to establish the diagnosis, the site of duodenal injury, possible cause, management of complication and its outcome.
The model (fixed or random effect) for meta analyses was selected, based Q and I2 statistics. STATA software was used to draw the forest plot and to compute the overall estimate and the 95%CI for the time of detection of injury and mortality. The association between time of detection of injury and mortality was estimated using χ2 test with Yate’s correction. Based on Kaplan Meier survival curve concept, the cumulative survival probabilities at various days of injury was derived.
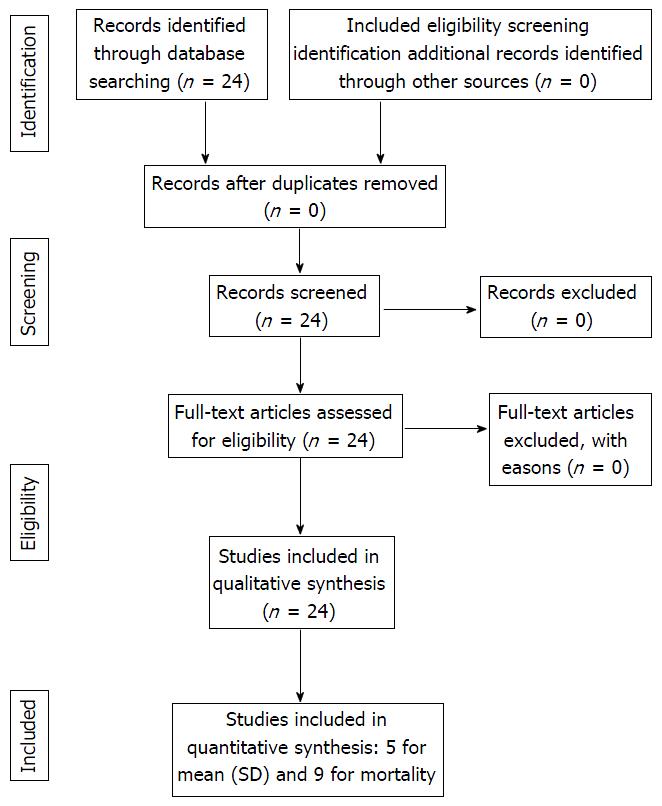
The PRISMA flow chart is presented in Figure 1. There were 24 studies (case report, case series) identified from the literature. None of the study was excluded. All 24 studies have been considered for both qualitative and quantitative evaluation.
A total of 74 cases of duodenal injuries were identified. Unfortunately details regarding the demography, presentation, investigations, management and outcome were not reported in several studies, particularly those reporting on overall complications of LC. Among the 24 cases with demography details, 11 patients (46%) were males and 13 patients (54%) were females, with a mean age of 58 years (range - 23 to 80 years) (Table 1). The mean period of detection of all the injury in the post-operative period was 1.7 d (range-immediate on table to 9th postoperative day). In 26 patients where the details of time of injury were noted, 12 (46%) of them were detected immediately on the table (day 0). Among the 28 cases where the cause of injury was reported, 13 (46%) occurred due to cautery, 11 (39%) during dissection, 4 (14%) due to retraction. There were no reported cases of injury due to veress needle or trocar insertion (Table 2). The presentation ranged from abdominal pain, nausea vomiting, abdominal tenderness, guarding, fever, peritonitis, bile drainage from the drainage tube, peritonitis, intra-abdominal abscess, sepsis and septic shock. Investigations that facilitated diagnosis included computed tomography (CT) abdomen, ultrasound with or without aspiration, estimation of amylase level in the drainage/aspirated fluid, gastrograffin study and gastroscopy and diagnostic laparoscopy (Table 1). The site of injury was reported in 13 cases of which 4 cases (31%) occurred in the 1st part, 6 cases (46%) occurred in the 2nd part above the duodenal papilla, 2 cases (15%) occurred in the 2nd part (below the papilla) and 1 case (7.6%) occurred in the 3rd part of the duodenum. Among the 30 cases where the management was reported, 19 (63%) underwent primary closure of duodenal perforation (duodenorapphy) among which 4 (21%) was carried out laparoscopically. The remaining procedures included percutaneous drainage in 4 cases (13.3%), tube duodenostomy (6.6%), gastric resection 2 cases (6.6%), and one each (3.3%) of Whipple resection, pyloric exclusion with gastrojejunostomy and laparoscopic endo gastrointestinal (GI) closure of perforation (Table 2). Among the 65 cases where the outcome was defined, 53 patients (82%) survived and 12 patients (18%) died (Table 2). The mean period of detection of injury in those who survived was 1.6 d (including those detected on table considered as day 0 compared to 4.25 d in those who died). However, if the ones who were detected on the table were excluded, the mean detection time for those who survived was 2.36 d compared 4.25 d for those who died.
| Ref. | Nature of study | No. of DuI/No. of LC (%) | Age (yr)mean range | Gender | Time of diagnosis From LC in days | Presentation | Investigations |
| Modi et al[8] | CR | 1 | 47 | M | 2 | BDr-1 | CT-1 |
| Fist-1 | |||||||
| Gasc-1 | |||||||
| Jing et al[9] | CR | 1 | 74 | M | 4 | Fever | CT-1 |
| BA-St-1 | |||||||
| US-asp-1 | |||||||
| Yajima et al[26] | CS | 1/407 | NA | NA | Immed-day 0 | NA | NA |
| Testini et al[6] | CS | 5 | 59 (49-51) | M-4 | Immed-1-day 0 | Fever-2 | Amyd-1 |
| F-1 | 1st day-1 | Tachycardia-1 | CT-4 | ||||
| 3rd day-1 | Leukocytosis | ||||||
| 4th day-1 | Rigidity/vomiting | ||||||
| 5th day-1 | Sshock-2 | ||||||
| Singh[10] | CS | 3/1748 (0.17%) | 33 (23-45) | F-3 | Immed-2 | Fever | CT-2 |
| 2nd day-1 | Abd. pain | ||||||
| Avrutis et al[24] | CR | 1 | NA | NA | 9th day | Haemtemesis | NA |
| Intra-abd abscess | |||||||
| Kwon et al[11] | CS | 2/1190 | 50 | M-2 | NA | NA | NA |
| 68 | |||||||
| El-Banna et al[7] | CS | 4/NA | 32-73 | M = 2 | Immed-1-day 0 | Sshock-2 (4 d) | X-ray abd |
| 47 | F = 2 | 3rd day-1 | Local peritonitis-1 | CT | |||
| 4th day-2 | Diffuse peritonitis-1 | US abd | |||||
| Bishoff et al[30] | CS | 1/915 | NA | NA | NA | NA | NA |
| Croce et al[12] | CS | 4/2100 (0.2%) | 50 (45-56) | M = 1 | Immed = 2 -day 0 | Abd pain = 4 | US asp-2 |
| F = 3 | 2nd day = 2 | Tachycardia leukocytosis | CT = 2 | ||||
| Rigidity = 1 vomiting | Gastrffin = 2 | ||||||
| Amyd = 2 | |||||||
| Relapsc = 4 | |||||||
| Roviaro et al[13] | CS | 1/1005 (0.09%) | NA | NA | NA | NA | NA |
| Huang[14] | CS | 19/39238 (0.04%) | NA | NA | NA | NA | NA |
| Wherry et al[2] | CS | 4/9130 (0.04%) | NA | NA | NA | NA | NA |
| Schrenk et al[15] | CS | 2/1690 (0.1%) | 70 80 | F = 2 | Immed = 2 –day 0 | NA | NA |
| Chen et al[17] | CS | 1/2428 (0.04%) | NA | NA | Immed = 1 -day 0 | Immed = 1 | Immed = 1 |
| Kum et al[16] | CS | 1/25 (4%) | NA | NA | Immed = 1 –day 0 | Immed = 1 | Immed = 1 |
| Cala et al[19] | CS | 1/1000 (0.01%) | NA | NA | NA | NA | NA |
| Baev et al[20] | CS | 1/700 (0.14%) | NA | NA | NA | NA | NA |
| Yamashita et al[21] | CS | 1/1054 (0.09%) | 42 | F | Immed = -day 0 | Immed = 1 | Immed = 1 |
| Berry et al[25] | CR | 1 | 76 | F | 6th day | Tachycardia, tachypnea, nausea, vomiting, leukocytosis | CT |
| Ward et al[18] | CS | 1/NR | NA | NA | NA | NA | NA |
| Ress et al[4] | CS | 3/NA | NA | NA | Immed = 1 -day 0 | Sshock = 1 | CT |
| Ist day = 1 | Abd pain = 1 | US | |||||
| 4th day = 1 | Immed = 1 | ||||||
| Deziel et al[5] | CS | 12/77.604 (0.01%) | NA | NA | NA | NA | NA |
| Peters et al[23] | CS | 2/283 (0.7%) | NA | NA | NA | NA | NA |
| Series | Site of injury | Cause of injury | Nature of surgery | Day of detection and outcome |
| Modi et al[8] | D1/D2 junction | BlDis-1 | Conservative - US guided aspiration of collection = 1 | 2nd day - survived |
| Jing et al[9] | D1 = diverticulum | NA | Conservative - percutaneous drain = 1 | 4th day - survived |
| Yajima et al[26] | NA | NA | NA | NA |
| Testini et al[6] | D2A-2 | BlDis-2 | Duodenorapphy + t tube = 2 | Immed-day 0 - survived |
| D2B-2 | Caut-3 | Petzer t tube = 1 | 1st day = survived | |
| D3-1 | Gastric resection = 1 | 3rd day = survived | ||
| Whipple resection = 1 | 4th day = survived | |||
| 5th day = died | ||||
| Singh et al[10] | NA | Bl Dis-3 | Duodenorapphy = 3 | Immed-day 0 - survived |
| Immed-day 0 - survived | ||||
| 2nd day = survived | ||||
| Avrutis et al[24] | NA | NA | NA | NA |
| Kwon et al[11] | NA | Bl Dis-2 | Laparoscopic Endo GI closure = 1 | |
| Laparoscopic intracorporeal suturing = 1 | Day of injury = NA | |||
| Survived | ||||
| El-Banna et al[7] | NA | Caut-4 | Precutaneous drain = 1 | Immed-day 0 - died |
| Gastrectomy + duodenostomy = 2 | 3rd day = survived | |||
| Serosal patch = 1 | 4th day = died | |||
| 4th day = died | ||||
| Bishoff et al[30] | NA | Bl Dis- scissors | Laparotomy duodenorapphy = 1 | NA |
| Croce et al[12] | D1 = 2 | Retraction = 3 | Laparoscopic - intracorporeal suturing = 2 | Immed-day 0 = survived |
| D2 = 2 | Caut = 1 | Laparotomy = duodenorapphy + omental patch | Immed-day 0 = survived | |
| Relaproscopy = missed injury, conservative (NPO/TPN/somataostatin) = 1 | 2nd day = survived | |||
| 2nd day = survived | ||||
| Roviaro et al[13] | NA | NA | NA | NA |
| Huang et al[14] | NA | NA | NA | NA |
| Wherry et al[2] | NA | NA | NA | Day of injury = NA |
| Survived = 3 | ||||
| Died = 1 | ||||
| Schrenk et al[15] | NA | Caut = 1 | Duodenorapphy = 2 | Immed-day 0 = survived |
| Bl Dis = 1 | Immed-day 0 = survived | |||
| Chen et al[17] | NA | NA | NA | NA |
| Kum et al[16] | NA | Caut = 1 | Laparoscopy + duodenorapphy = 1 | Immed-day 0 = survived |
| Cala et al[19] | NA | NA | NA | NA |
| Baev et al[20] | NA | NA | Laparotomy + duodenorapphy = 1 | NA |
| Yamashita et al[21] | NA | Retraction | Laparotomy + duodenorapphy = 1 | Immed-day 0 = survived |
| Berry et al[25] | D2A | Caut = 1 | T tube duodenostomy + pyloric exclusion + gastrojejunostomy | 6th day = survived |
| Ward et al[18] | NA | NA | NA | NA |
| Ress et al[4] | NA | Caut = 2 | Laparoscopy + serosal tear repair | Immed = day 0 = survived |
| Bl dis = 1 | Laparotomy + duodenorapphy = 2 | Ist day = 1 = survived | ||
| 4th day = 1 = died | ||||
| Deziel et al[5] | NA | NA | Laparotomy = 12 (details NA) | Day of injury = NA |
| Survived = 11 | ||||
| Died = 1 | ||||
| Peters et al[23] | NA | NA | NA | NA |
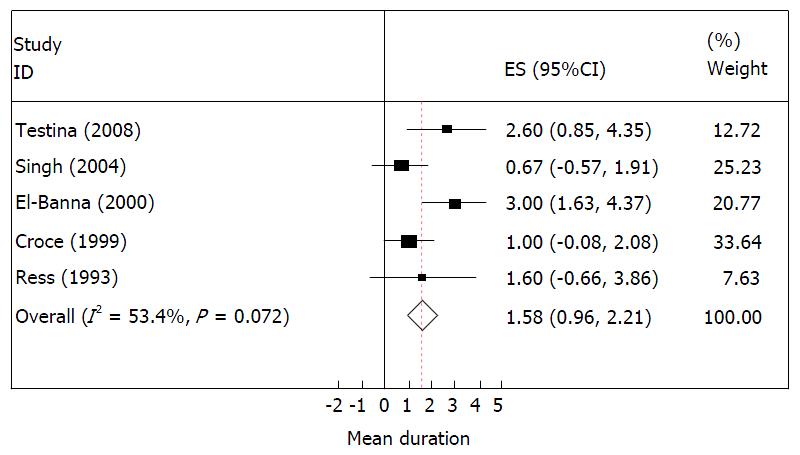
The forest plot for time of detection of injury (in days) is presented in Figure 2. This suggests that there is no heterogeneity among studies considered in the analyses as the I2 was about 50%. Therefore, based on the random effect model the overall mean (95%CI) duration of detection of injury is about 1.6 (1.0, 2.2) d.
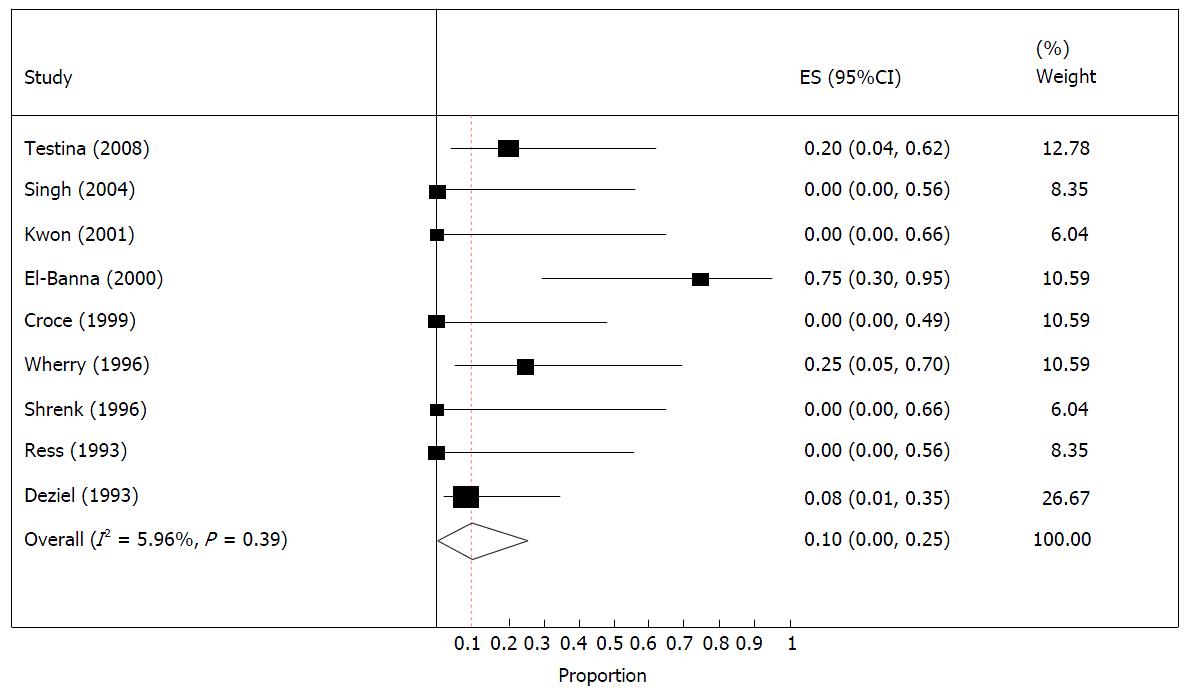
The forest plot for injury related mortality is provided in Figure 3. This also suggests that there is no heterogeneity among studies, as the I2 was about 6%. Therefore, based on the fixed effect model the overall mortality rate from these studies was 10% (0%, 25%).
Table 3 presents the mortality status according to days of detection. There were 15 patients who were detected to have injury within a day (0 or 1 d). Of them one patient died (6.7%). However, of the 13 patients who were detected to have injury after day 1, 5 of them have died (38.5%). The difference in mortality rate was significantly different suggesting that early detection was associated with lower mortality (P < 0.05).
| Days of detection of injury | Injury detection status | Total | |||
| Alive | Dead | ||||
| n | % | n | % | ||
| 0 | 12 | 100 | 0 | 0.0 | 12 |
| 1 | 2 | 66.7 | 1 | 33.3 | 3 |
| 2 | 2 | 50.0 | 2 | 50.0 | 4 |
| ≥ 3 | 6 | 66.7 | 3 | 33.3 | 9 |
| Total | 22 | 78.6 | 6 | 21.4 | 28 |
Based on the Kaplan Meier survival probabilities for mortality of patients over time (days), the cumulative probability of survival was about 94%, if the injury was detected on day 1 and 80%, if the injury was detected at day 2. However, if the injuries were detected later on, then the survival probabilities dropped down steeply, especially after day 2 (Figure 4). This suggests that if the day of detection is delayed then the probability of dying is very high.
LC is the standard treatment for symptomatic cholelithiasis. Over the years, difficult LC has been conducted regularly even in patients with active inflammation, cirrhosis, adhesions and contracted fibrosed gall bladder[1,6,28,29]. This has been possible due to the growing experience in laparoscopic surgery and advances made in instrumentation[28,29]. Unfortunately, major complications including bowel injuries still occur, and duodenal injury among them though rare, are generally associated with significant morbidity and mortality[4-26].
Major complications following LC have been reported in 2% of large series among which bowel injuries occurred in 0.07%-0.9% of these cases[15]. Among the bowel injuries, 58% occurred in small bowel, 32% in large bowel and 7% in stomach[28]. The overall incidence of duodenal injuries is reported to be 0.04% (range 0.01%-4%)[6]. While bile duct injury is the most common, vascular and bowel injuries are the most serious procedure related complications[3,5,7,28-30].
Injuries to the bowel could be related to the introduction of a Veress needle, trocar insertion, application of grasping forceps, sharp dissection with scissors and thermal contact burns or conductive burns during a laparoscopic procedure[6,30]. In a large study of 226 bowel injuries sustained during 205969 cases of laparoscopic procedures, 50% were caused by cautery and 32% by Veress needle or trocar insertion[30]. In another larger study of 430 bowel injury following 329935 cases of laparoscopic procedures, small intestine injury occurred in 55.8%, followed by large intestine (38.65%)[31]. Importantly, 66.8% of bowel injuries were diagnosed during laparoscopy or within 24 h thereafter.
A trocar or Veress needle insertion caused 41.8% of bowel injuries and those due to coagulator or laser were in 25.6% of the cases[31]. Bowel injuries resulting from trocar puncture is usually readily recognized and promptly repaired[6]. Bowel injuries related to Veress needle or trocar insertion may have declined over the years[11]. Duodenum being anatomically retroperitoneal and away from the umbilicus (the usual site of access for pneumoperitoneum) is unlikely to sustain injury during initial step of insufflation[25]. This is reflected in this review, were no cases of duodenal injury have been reported due to veress needle or trocar insertion.
Duodenal injury is more likely to occur due to thermal injury, sustained during the use of cautery[6,30,32]. It was noted as a leading cause in 46% of the cases in this review. It is at risk of being overlooked in the course of surgery and may manifest itself later as a consequence of coagulation necrosis of the bowel wall[2,5,15,31]. Bowel wall necrosis may result in delayed or walled of perforation, which may present in days or weeks[12,15,32]. Majority of the laparoscopic duodenal injuries reported in the literature and found in our review are due to electrocautery damage, or during the dissection of difficult Calot’s triangle, either due to adhesions or because of the distorted anatomy[10,12,14,16,32]. To prevent thermal injury, the equipment should be checked regularly for defects in insulation[4,15]. In addition, movements of all instruments should be under direct vision by following it with camera, while the instruments are out of view[15]. Others have suggested avoiding the use of sharp pointed suction/irrigation devices to retract the duodenum[12]. The sharp edge of the suction device may traumatize the duodenum, when used to retract it caudally and to the left[12]. When bowel has been grasped during manipulation, the site that is grasped is carefully inspected for any possible injury, particularly when the gut is unusually vulnerable for injury[15]. Inadvertent bowel retraction, along with injury during the use of electrocautery is often the cause of duodenal injury[4-6,10,14,15] (Table 2). Thermal burns can to a large extent be reduced by ensuring adequate insulation up to its tips, use of low power current, and nonuse of cautery in close proximity to the bowel. It should be rather used directly on tissues to be cauterized[15]. One should also be aware of the capacitative coupling that occurs along the shaft of instruments, with relatively thin insulation coats[33]. This stray energy may be responsible for otherwise unrecognized, unintentional injury during monopolar laparoscopic cauterization[15,23,33].
The risk of complication during surgery is often reported to be related to surgeons experience; however, experienced surgeons often attempt to operate under less than ideal circumstances and in complex situations[5,6,30]. In one of the reports, 60% of bowel injuries occurred with surgeons who were experienced and would have performed at least 100 LC[6].
Time at which bowel injury is recognized following the laparoscopic procedure is variable and is reported to range from 2 to 14 d (average 4.5 d) for small bowel injury and from 1 to 29 d (average 5.4 d) for large bowel injury[30]. Duodenal injury may be detected on table or in the postoperative period [range 0 (on table) to 5 d] and is detected according to some report on an average on the 3rd postoperative day[6]. However, this review noted the detection rate on an average at 1.7 d as in 46% of the cases it was detected on the table. Diagnosis of duodenal injury in postoperative period is often difficult and requires a high index of clinical suspicion, because of its rarity[5,6,14,15,30]. Patients who had a difficult cholecystectomy due to adhesions of the gall bladder, particularly to the duodenum, are at a greater risk[10,14,15]. The injury should be suspected in patients with unexplained cause of postoperative fever, nausea, vomiting, anorexia and abdominal distension[5,6,12,14,15]. Pain, which may be undue and restricted initially to right hypochondrium, may later become generalized[12,14,15]. Pain in the early stages is likely to be ignored as it is a relatively common finding after LC. However, it becomes significant, if it persists beyond 24 h and increases in intensity[12]. Posterior wall duodenal perforation may not result in peritonitis, but may present with lumbar pain[12].
Liver function tests may be normal or show mild elevation of bilirubin and serum amylase with normal alkaline phosphatase[5,12,14,15]. The diagnosis however can be clinched, if the drain fluid shows high amylase levels, in patients where drain was placed intraoperatively, because of difficult cholecystectomy[12]. The amylase level could also be estimated by ultrasound guided aspiration of fluid of the duodenal leak[12]. When carried out, contrast study with gastrograffin may confirm the leak[12]. CT scan which is more sensitive than ultrasound abdomen, could reveal large collection of fluid around the duodenum or in the general peritoneal cavity, based on when the procedure is performed in the post operative period[6]. The finding of significant amount of air and fluid in the abdomen, beyond what can be explained as a postoperative finding and the demonstration of contrast leak when performed with oral contrast[9], are findings that are consistent with the diagnosis of duodenal injury[12]. Obliteration of the right psoas muscle, evidenced by retroperitoneal gas, may indicate retroperitoneal duodenal leak. When in doubt, it is advisable to perform at least an early diagnostic laparoscopy, as time is of essence for a better outcome[5,6,12,15]. Presence of bile on re-exploration, in the absence of leak from hepatic bed, cystic duct or common bile duct suggests the diagnosis of duodenal injury[12]. Forward displacement of the duodenum by posterior mass, reflects the posterior location of the perforation[23,25]. Unfortunately, laparoscopy my also fail in detecting a small perforation and this misdiagnosis may lead to intra abdominal or retroperitoneal collection in the lumbar region and sepsis leading to a protracted postoperative course[12]. In the event the injury is not obvious during laparoscopy, then it would be worthwhile detecting the injury by upper GI endoscopy and demonstrating air leak around the duodenum by air insufflation.
The outcome of duodenal injury would depend to a large extent on the site and the time of diagnosis[4-23]. The management could range from conservative in selected few[8,9], to more complex surgeries in those with delayed intervention[6,34,35]. While there are reports of successful conservative management[8,9], most would agree on an immediate surgical intervention[5,6,10,14,15,30]. Successful conservative management with drain has been reported in a patient with previous Billroth 11 gastrectomy[9]. This patient had sustained a cautery induced perforation to a duodenal bulb diverticulum, rather than the duodenal wall. The site of perforation and diversion of gastric contents is reported to have attributed to the successful conservative management in this patient[9]. Successful conservative management has also been reported in a patient where the drain that was inserted during the surgery, had inadvertently fistulated into the duodenal injury[8]. The drain was used successfully to divert the duodenal content in postoperative period, allowing the patient to respond to conservative management[8].
In those patients where surgical intervention is required, its nature would depend on the time of detection of injury and the site[5,6,14,15,30]. Duodenal perforation may require meticulous search, by means of intraoperative upper GI endoscopy or duodenal mobilization by Kocher’s maneuver[12]. When the injury is detected on table or following re-exploration shortly after LC, direct repair of duodenal injury with omental patch is feasible[6]. This repair could be performed laparoscopically, when the duodenum is relatively healthy, defect is small and expertise is available[11,12,16,22]. However, most recommend immediate laparotomy to assess the abdomen and secure a safe repair[4,5-7,21]. However, delay in diagnosis beyond 48 h may lead to oedematous macerated duodenum, which will fail to hold sutures of repair, resulting in duodenal fistula[5,6,10,14,15,30].
Site of duodenal injury is a critical factor, that influences both the outcome and approach to management[5-7,10,14,15,30]. When injury occurs just above or below the duodenal ampulla of vater, the biliary fluid and pancreatic juice leak will complicate matter[5,6,10,14,15]. Resection of the damaged tissue and repair could be challenging in these cases, particularly in patients where there is a delay in diagnosis. Several approaches have been proposed in the literature, which include mucosal or serosal patches and a pedicle graft with a free vascular pedicle created from stomach, jejunum or ileal tissue; however their efficacy has not been proven[36-38]. In general, the often practiced approach includes duodenal drainage with a decompression tube, temporary pyloric exclusion, gastrojejunostomy, feeding jejunostomy, gastric resection with external duodenal drainage with Foley or Petzer tubes[6]; however, the outcome reported are conflicting[34,39,40]. More aggressive approach may be warranted in the presence of larger defects and softer duodenal wall and may involve duodenojejunostomy or duodenopancreatectomy[6,23,34]. The outcome depends to a large extent on the degree of peritonitis and sepsis, which in turn is related to the extent of delay in diagnosis[5,6,10,14,15,29]. While the injury to descending duodenum is challenging to manage, those that occur at the duodenal bulb or superior flexure of duodenum, could be safely managed with gastric resection and duodenal stump closure[5,6]. Majority of the patients in this review underwent duodenorapphy or duodenostomy. In exceptional case, a patient may undergo Whipple resection[6]. In this review, in a solitary case, Whipple resection was carried out (Table 2). The injury was detected on the 4th postoperative day. While the need for pancreaticoduodenectomy is not clear, the gravity of the problem is reflected by the fact that the patient had a stormy postoperative period and was discharged two months later[6].
The concerning aspect of duodenal injuries is the reported mortality in the range of 8.3%[5] to 75%[7]. Deziel et al[5] reported an 8.3% mortality rate among 12 patients with duodenal injuries in their analysis of 77604 cases. El-Banna et al[7] noted mortality in three of the four (75%) duodenal injuries. Huang et al[14] reported that 4 out of 19 (21.05%) patients with duodenal injury died in their study of 39238 LC cases. Our review observed an overall mortality of 17%. It is most likely that the duodenal injuries are underreported[6,41]. These patients are also at the risk of having significant morbidity, which could lead to protracted hospital course[6]. The morbidity includes intra-abdominal complications like abscess and peritonitis[12], septicaemia, necrotising fasciitis[6,31], pneumonia[15], incisional hernia[7] and lumbar abscess[12] (Table 2). Posterior lumbar abscess may occur due to disruption of the posterior peritoneal membrane during cholecystectomy or during reoperation for duodenal repair[12].
Duodenal injury is uncommon but is associated with significant morbidity and mortality. These are sustained during LC, usually due to thermal burn and blunt or sharp dissection. Unsatisfactory recovery post difficult LC, should raise the suspicion. Radiological imaging, analysis of the drain fluid for bile and or amylase levels and endoscopy, will facilitate the diagnosis. Early diagnostic laparoscopy is warranted when in doubt. Prompt surgical intervention, which may involve duodenal repair or resection may be required. Outcome would be significantly influenced by the delay in diagnosis.
I would like to thank Professor Jeyaseelan L, Department of Statistics and Health Information, Sultan Qaboos University Hospital, Muscat, Oman for the statistical analysis and the figures.
Inadvertent duodenal injury is a rare but potentially fatal complication of laparoscopic cholecystectomy (LC). Such injuries often go unrecognized at the time of the procedure and manifest later with significant morbidity and mortality. In this article the literature is reviewed regarding the mechanism, presentation, investigation and management of this serious, though uncommon complication. Among the 76 cases that were detected in the literature, 46% of the injury was caused by the use of cautery and in 39% during dissection. The commonest site of injury was to the 2nd part of the duodenum and in only half of these patients, the injury was detected on table. Predominant repair was duodenorapphy and in 21% this was carried out laparoscopically. The mean day of detection was 1.6 d among those who survived compared to 4.25 d among those who died. Mortality of 18% was noted. This article is of importance as literature lacks adequate data on the etiopathogenesis, management and outcome of this rare, yet life threatening complication. Early detection requires high index of clinical suspicion in a patient with difficult cholecystectomy who has unexpected post operative course, raised amylase levels in fluid from the drain when placed or radiological images suggestive of subhepatic fluid collection not explained otherwise.
This article reviews the literature with regards to duodenal injury post LC. Review of literature indicates the commonest cause for injury is due to cautery and blunt and sharp dissection employed during cholecystectomy. The predominant finding is, that delay in diagnosis makes simple repair with duodenorapphy non feasible requiring more complex surgery. In addition the poor outcome is directly related to the delay in diagnosis.
This is a review article on duodenal injury post LC and aspects of innovations and breakthroughs may not be applicable to it.
This article is of importance to surgeons who perform LC. Its applicability is in warning clinicians of this potential complication when their patient develops postoperative abdominal pain and distension unexplained by any other cause. It then guides them in investigating these patients and managing them, while reminding them of the potential mechanism for this complication.
This is a good review of an uncommon condition.
P- Reviewer: Farkas DT, Kirshtein B, Ozdemir F S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Agresta F, Campanile FC, Vettoretto N, Silecchia G, Bergamini C, Maida P, Lombari P, Narilli P, Marchi D, Carrara A. Laparoscopic cholecystectomy: consensus conference-based guidelines. Langenbecks Arch Surg. 2015;400:429-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Wherry DC, Marohn MR, Malanoski MP, Hetz SP, Rich NM. An external audit of laparoscopic cholecystectomy in the steady state performed in medical treatment facilities of the Department of Defense. Ann Surg. 1996;224:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Machado NO. Biliary complications postlaparoscopic cholecystectomy: mechanism, preventive measures, and approach to management: a review. Diagn Ther Endosc. 2011;2011:967017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Ress AM, Sarr MG, Nagorney DM, Farnell MB, Donohue JH, McIlrath DC. Spectrum and management of major complications of laparoscopic cholecystectomy. Am J Surg. 1993;165:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 774] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 6. | Testini M, Piccinni G, Lissidini G, Di Venere B, Gurrado A, Poli E, Brienza N, Biondi A, Greco L, Nacchiero M. Management of descending duodenal injuries secondary to laparoscopic cholecystectomy. Dig Surg. 2008;25:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | El-Banna M, Abdel-Atty M, El-Meteini M, Aly S. Management of laparoscopic-related bowel injuries. Surg Endosc. 2000;14:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Modi M, Deolekar S, Gvalani A. An option of conservative management of a duodenal injury following laparoscopic cholecystectomy. Case Rep Surg. 2014;2014:398545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Jing K, Shuo-Dong W. Postoperative Delayed Duodenum Perforation following Elective Laparoscopic Cholecystectomy. Case Rep Med. 2014;2014:823149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Singh R, Kaushik R, Sharma R, Attri AK. Non-biliary mishaps during laparoscopic cholecystectomy. Indian J Gastroenterol. 2004;23:47-49. [PubMed] |
| 11. | Kwon AH, Inui H, Kamiyama Y. Laparoscopic management of bile duct and bowel injury during laparoscopic cholecystectomy. World J Surg. 2001;25:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Croce E, Golia M, Russo R, Azzola M, Olmi S, De Murtas G. Duodenal perforations after laparoscopic cholecystectomy. Surg Endosc. 1999;13:523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Roviaro GC, Maciocco M, Rebuffat C, Varoli F, Vergani V, Rabughino G, Scarduelli A. Complications following cholecystectomy. J R Coll Surg Edinb. 1997;42:324-328. [PubMed] |
| 14. | Huang X, Feng Y, Huang Z. Complications of laparoscopic cholecystectomy in China: an analysis of 39,238 cases. Chin Med J (Engl). 1997;110:704-706. [PubMed] |
| 15. | Schrenk P, Woisetschläger R, Rieger R, Wayand W. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kum CK, Eypasch E, Aljaziri A, Troidl H. Randomized comparison of pulmonary function after the ‘French’ and ‘American’ techniques of laparoscopic cholecystectomy. Br J Surg. 1996;83:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Chen XR, Lou D, Li SH, Mao JX, Zhou ZD, Yu SM, Duan ZW. Avoiding serious complications in laparoscopic cholecystectomy--lessons learned from an experience of 2428 cases. Ann Acad Med Singapore. 1996;25:635-639. [PubMed] |
| 18. | Ward EM, LeRoy AJ, Bender CE, Donohue JH, Hughes RW. Imaging of complications of laparoscopic cholecystectomy. Abdom Imaging. 1993;18:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Cala Z, Velnić D, Cvitanović B, Rasić Z, Perko Z. Laparoscopic cholecystectomy: results after 1000 procedures. Acta Med Croatica. 1996;50:147-149. [PubMed] |
| 20. | Baev S, Pozarliev T, Todorov GT. Laparoscopic cholecystectomy: 700 consecutive cases. Int Surg. 1995;80:296-298. [PubMed] |
| 21. | Yamashita Y, Kurohiji T, Kakegawa T. Evaluation of two training programs for laparoscopic cholecystectomy: incidence of major complications. World J Surg. 1994;18:279-285; discussion 285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Taylor AM, Li MK. Laparoscopic management of complications following laparoscopic cholecystectomy. Aust N Z J Surg. 1994;64:827-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Peters JH, Gibbons GD, Innes JT, Nichols KE, Front ME, Roby SR, Ellison EC. Complications of laparoscopic cholecystectomy. Surgery. 1991;110:769-777; discussion 777-778. [PubMed] |
| 24. | Avrutis O, Meshoulam J, Yutkin O, Mikchalevski V, Haskel L, Adler S, Durst A. Brief clinical report: duodenal laceration presenting as massive hematemesis and multiple intraabdominal abscesses after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2001;11:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Berry SM, Ose KJ, Bell RH, Fink AS. Thermal injury of the posterior duodenum during laparoscopic cholecystectomy. Surg Endosc. 1994;8:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Yajima H, Kanai H, Son K, Yoshida K, Yanaga K. Reasons and risk factors for intraoperative conversion from laparoscopic to open cholecystectomy. Surg Today. 2014;44:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Wolfe BM, Gardiner BN, Leary BF, Frey CF. Endoscopic cholecystectomy. An analysis of complications. Arch Surg. 1991;126:1192-1196; discussion 1196-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Zafar SN, Obirieze A, Adesibikan B, Cornwell EE, Fullum TM, Tran DD. Optimal time for early laparoscopic cholecystectomy for acute cholecystitis. JAMA Surg. 2015;150:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Machado NO. Laparoscopic cholecystectomy in cirrhotics. JSLS. 2012;16:392-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Bishoff JT, Allaf ME, Kirkels W, Moore RG, Kavoussi LR, Schroder F. Laparoscopic bowel injury: incidence and clinical presentation. J Urol. 1999;161:887-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | van der Voort M, Heijnsdijk EA, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg. 2004;91:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Z’graggen K, Wehrli H, Metzger A, Buehler M, Frei E, Klaiber C. Complications of laparoscopic cholecystectomy in Switzerland. A prospective 3-year study of 10,174 patients. Swiss Association of Laparoscopic and Thoracoscopic Surgery. Surg Endosc. 1998;12:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Grosskinsky CM, Hulka JF. Unipolar electrosurgery in operative laparoscopy. Capacitance as a potential source of injury. J Reprod Med. 1995;40:549-552. [PubMed] |
| 34. | Carrillo EH, Richardson JD, Miller FB. Evolution in the management of duodenal injuries. J Trauma. 1996;40:1037-1045; discussion 1045-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Cogbill TH, Moore EE, Feliciano DV, Hoyt DB, Jurkovich GJ, Morris JA, Mucha P, Ross SE, Strutt PJ, Moore FA. Conservative management of duodenal trauma: a multicenter perspective. J Trauma. 1990;30:1469-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | DeShazo CV, Snyder WH, Daugherty CG, Crenshaw CA. Mucosal pedicle graft of jejunum for large gastroduodenal defects. Am J Surg. 1972;124:671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Walley BD, Goco I. Duodenal patch grafting. Am J Surg. 1980;140:706-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Talving P, Nicol AJ, Navsaria PH. Civilian duodenal gunshot wounds: surgical management made simpler. World J Surg. 2006;30:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Snyder WH, Weigelt JA, Watkins WL, Bietz DS. The surgical management of duodenal trauma. Precepts based on a review of 247 cases. Arch Surg. 1980;115:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ivatury RR, Gaudino J, Ascer E, Nallathambi M, Ramirez-Schon G, Stahl WM. Treatment of penetrating duodenal injuries: primary repair vs. repair with decompressive enterostomy/serosal patch. J Trauma. 1985;25:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Voyles CR, Tucker RD. Education and engineering solutions for potential problems with laparoscopic monopolar electrosurgery. Am J Surg. 1992;164:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |