Published online Mar 27, 2016. doi: 10.4240/wjgs.v8.i3.252
Peer-review started: July 2, 2015
First decision: August 25, 2015
Revised: December 24, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: March 27, 2016
Processing time: 265 Days and 10.9 Hours
AIM: To provide an update on the aetiology, pathogenesis, diagnosis, staging and management of rectal squamous cell carcinoma (SCC).
METHODS: A systematic review was conducted according to the preferred reporting items for Systematic Reviews and Meta-Analyses guidelines. A comprehensive search of Ovid MEDLINE was performed with the reference list of selected articles reviewed to ensure all relevant publications were captured. The search strategy was limited to the English language, spanning from 1946 to 2015. A qualitative analysis was undertaken examining patient demographics, clinical presentation, diagnosis, staging, treatment and outcome. The quantitaive analysis was limited to data extracted on treatment and outcomes including radiological, clinical and pathological complete response where available. The narrative and quantitative review were synthesised in concert.
RESULTS: The search identified 487 articles in total with 79 included in the qualitative review. The quantitative analysis involved 63 articles, consisting of 43 case reports and 20 case series with a total of 142 individual cases. The underlying pathogenesis of rectal SCC while unclear, continues to be defined, with increasing evidence of a metaplasia-dysplasia-carcinoma sequence and a possible role for human papilloma virus in this progression. The presentation is similar to rectal adenocarcinoma, with a diagnosis confirmed by endoscopic biopsy. Many presumed rectal SCC’s are in fact an extension of an anal SCC, and cytokeratin markers are a useful adjunct in this distinction. Staging is most accurately reflected by the tumour-node-metastasis classification for rectal adenocarcinoma. It involves examining locoregional disease by way of magnetic resonance imaging and/or endorectal ultrasound, with systemic spread excluded by way of computed tomography. Positron emission tomography is integral in the workup to exclude an external site of primary SCC with metastasis to the rectum. While the optimal treatment remains as yet undefined, recent studies have demonstrated a global shift away from surgery towards definitive chemoradiotherapy as primary treatment. Pooled overall survival was calculated to be 86% in patients managed with chemoradiation compared with 48% for those treated traditionally with surgery. Furthermore, local recurrence and metastatic rates were 25% vs 10% and 30% vs 13% for the chemoradiation vs conventional treatment cohorts.
CONCLUSION: The changing paradigm in the treatment of rectal SCC holds great promise for improved outcomes in this rare disease.
Core tip: Primary squamous cell carcinoma (SCC) of the rectum is a rare entity with a historically poor prognosis. This systematic review provides an in depth summary of the current body of knowledge surrounding the aetiology, pathogenesis, diagnosis, staging and prognosis of this disease. Given the current paradigm shift in the first line treatment of rectal SCC away from traditional surgical management towards definitive chemoradiotherapy, the evidence supporting this change is examined.
- Citation: Guerra GR, Kong CH, Warrier SK, Lynch AC, Heriot AG, Ngan SY. Primary squamous cell carcinoma of the rectum: An update and implications for treatment. World J Gastrointest Surg 2016; 8(3): 252-265
- URL: https://www.wjgnet.com/1948-9366/full/v8/i3/252.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i3.252
Rectal squamous cell carcinoma (SCC) is a rare malignancy of the gastrointestinal (GI) tract. Due to the low incidence of this cancer and subsequent lack of literature, the underlying pathogenesis and risk factors are yet to be clearly defined. Furthermore, there is significant heterogeneity in the treatment regimens utilised, with the optimal management yet to be clarified. Nonetheless, certain patterns do emerge on reviewing all published cases by way of a systematic review, to determine where our future research should be directed in order to improve upon treatment and facilitate best patient outcomes.
A systematic literature review was conducted according to the preferred reporting items for Systematic Reviews and Meta-Analyses guidelines. A comprehensive search of Ovid Medline was performed with the abstracts screened to determine relevant articles, following which the full texts were obtained. A directed manual review of all embedded references was undertaken of the selected articles to ensure all studies published on primary SCC of the rectum were identified.
The search strategy was based on a combination of medical subject heading terms (carcinoma, squamous cell; rectum) and text words (SCC and rectum), spanning from 1946 to May 2015. The search was limited to English language with the most recent search performed on 8th May 2015.
The definition of rectal SCC as stipulated by Williams et al[1] which requires three exclusion criteria to be met (detailed in “diagnosis” below) was used to identify relevant studies. Consequently, studies reporting rectal SCC arising in the presence of a fistula, from an anal or gynaecological origin, a distant site via metastasis, or where the pathology was mixed (e.g., adenosquamous) were excluded. Additionally, studies where the lesion was premalignant (e.g., metaplasia or SCC in situ), of colonic rather than rectal origin or where the data was inadequate were excluded from the quantitative analysis.
Data extracted included the names of the authors, date of publication, demographic information and clinical presentation. Location of the lesion and treatment detailing the primary modality, the use of pre- and/or post-operative modalities and the type of operation where present was also noted. Other collated information included patient outcomes in the form of local recurrence, metastasis, and survival, as well as the length of follow up. Radiological, clinical and pathological complete response (CR) was also recorded where available.
The database and bibliography search identified 487 articles in total. After screening the articles for inclusion and exclusion criteria, 79 were included in the qualitative review and 63 in the quantitative analysis as detailed in Figure 1. This included 43 case reports and 20 case series with a total of 142 individual cases reported. Given the inherent bias in case reports and the inconsistency with reporting important prognostic variables including stage and pathological grade, an in depth individual patient data meta-analysis was not performed.
Epidemiology: Rectal SCC is a rare disease with the current literature consisting primarily of case reports, case series and one large population based study. While SCC can occur throughout the GI tract, it most commonly affects the upper aerodigestive tract down to the oesophagogastric junction, and the anal canal. SCC of the rectum however is much less common accounting for 0.3% of all histological subtypes[2]. While pure SCC is the most frequent histology, cases with a mixed histologic pattern, generally adenosquamous, have been described[3]. While other rectal cancer subtypes including neuroendocrine, lymphoma and gastrointestinal stromal tumours occur infrequently, rectal SCC remains the most rare with the exception of sarcoma[2].
Schmidtmann[4] reported the first case of SCC of the colon in 1919, with Raiford[5] publishing on the first case of rectal SCC in 1933. While SCC can be diagnosed throughout the colorectum, the most common site of predilection is the rectum (93.4%), followed by the right colon (3.4%)[2]. The true incidence of rectal SCC can be most accurately drawn from the large population based study from the National Cancer Institute (NCI), which estimated it at 1.9 per million population in the year 2000, or 3 per 1000 colorectal cancers. This study also identified a significant rise in the incidence of rectal SCC between 1992 and 2000, estimating it at 5.9% per year. Extrapolating from this figure, the current incidence may be as high as 3.5 per million population[2].
While strong epidemiological evidence on rectal SCC is absent, patient demographics and risk factors can be gauged from the published retrospective reviews and population study. Patients diagnosed with SCC of the rectum have ranged in age from 39 to 93 years old, with an average age of 63 years. Female gender predominates, accounting for 57.4% vs 42.6% of cases in the NCI study. Patients most frequently present with early stage localised (stage I/II, 52.8%) or regional (stage III, 29.3%) disease and there is no apparent ethnic or geographic predisposition[2].
Despite a lack of firm risk factors with a causal link to the development of rectal SCC, loose associations have been identified. The strongest association evident in the literature is that of proctitis, generally secondary to ulcerative colitis. There have been multiple case reports of rectal SCC in this setting, one of which compared the incidence with that of the general population to demonstrate a markedly increased risk in ulcerative colitis patients[6-15]. Of significance, there has also been a report of rectal SCC in the setting of active Crohn’s disease of the rectum[16], and in the setting of chronic prolapse[17]. Drawing upon this association with inflammation, the literature also contains three reports of parasitic infections with colorectal SCC, in the form of Schistosomiasis in two cases, and Amoebiasis in one, however, their significance is unclear[1,18,19].
Other postulated risk factors have included a past history of radiotherapy for other pelvic malignancies, which has been noted in several case reports[20-23]. Additionally, colorectal adenocarcinoma, both synchronous and metachronous has been identified in patients with SCC of the rectum[3,24-27]. For colonic SCC, asbestos exposure and colonic duplication have also been associated, but this has not been the case for SCC of rectal origin.
Given the strong association of human papilloma virus (HPV) with anal SCC, several studies have investigated its role in rectal SCC. This has produced variable results, with as many studies identifying HPV 16 in colorectal SCC specimens[12,17,28,29], as those that have failed[3,16,18]. Given this limited evidence, HPV infection as a risk factor for rectal SCC remains to be proven.
Pathogenesis: Despite reports of rectal SCC since the early 20th century, it’s underlying aetiology remains unclear. While multiple theories have been postulated over this time period, its pathogenesis continues to be unravelled by assimilating the current body of evidence.
The theory of chronic inflammation leading to squamous metaplasia and subsequent carcinoma is one of the most prominent. This idea draws upon the fact that irritation and inflammation can lead to a change in the epithelial lining. This is termed metaplasia and is known to occur in the GI tract in response to exposure to various stressors[30]. Metaplasia is the reversible change of one adult cell type into another and represents an adaptive substitution of stress-sensitive cells by a cell type better able to withstand that particular insult[31]. The postulated inciting cause for the chronic inflammation leading to metaplasia has included the risk factors mentioned above of ulcerative colitis[6,32], radiotherapy[14,20-23] and infection[18].
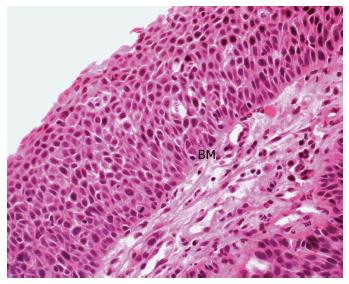
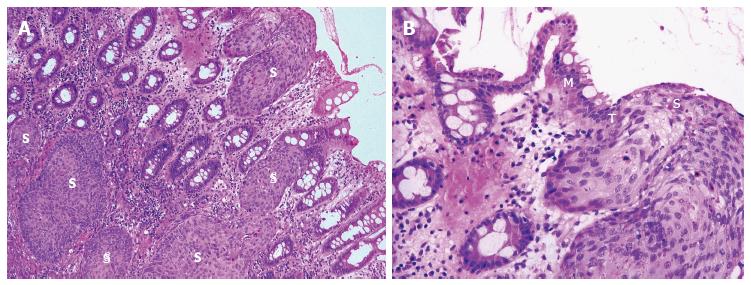
Adding support to this theory is firstly the description of squamous metaplasia in the colorectum in numerous instances. This has included sporadically[33-36], in the regenerating epithelium of chronic ulcerative colitis[15,32], in a rat by instillation of a chronic irritant (H2O2) and in a mouse secondary to chronic rectal prolapse[37,38]. Secondly and of most significance, is the demonstration of an adjacent histological sequence in the rectum, from squamous metaplasia through dysplasia to carcinoma in situ (Figure 2) and invasive squamous carcinoma (Figure 3)[6,7,12,15,24,35].
Drawing further upon this theory is the idea of pluripotent mucosal stem cells capable of multidirectional differentiation, first postulated in the 1950’s[17,39-41]. Further work by Nahas et al[16] in 2007 was based on the fact that keratin profiles vary amongst epithelia but remain constant in neoplastic transformation. They demonstrated that rectal SCC and adenocarcinoma stain for cytokeratin CAM5.2, unlike SCC of the anal margin, suggesting a common cell of origin for both rectal cancer subtypes. This lends support to an idea that the mucosal lining of the rectum contains a common pluripotent endodermal stem cell, which under certain conditions (inflammation and epithelial damage) can undergo squamous differentiation to better protect the rectum from the inciting cause. This is visualised as an area of metaplasia, which can subsequently undergo dysplasia and carcinomatous change if the inciting cause is not removed.
HPV has been postulated as a possible factor in inciting the dysplastic change of the squamous metaplasia. However, while there is a strong association between HPV and SCC of multiple sites including the anus, head/neck and cervix, the role in SCC of the rectum has not currently been established. There are more than 100 subtypes of HPV, with the most frequently encountered oncogenic forms being HPV 16 and 18. There are only a limited number of studies that have examined for HPV in rectal SCC, and they have utilised varying techniques for detection with discordant results. Audeau et al[18] used immunohistochemistry to examine 20 squamous lesions (squamous metaplasia, SCC, adenosquamous carcinoma), without evidence of HPV 6, 11, 16 or 18. Frizelle et al[3] and Nahas et al[16] used an in situ hybridisation technique on 6 and 5 rectal SCC specimens respectively, again without evidence of HPV deoxyribonucleic acid (DNA). However, studies by Sotlar et al[28] (1 rectal SCC), Kong et al[12] (2 rectal SCC, 1 rectal SCC in situ), Matsuda et al[29] (1 rectal SCC) and Jaworski et al[17] (2 rectal SCC in situ), all identified HPV 16 in 7 rectal squamous lesions when utilising the PCR method, which is regarded as the gold standard. This may indicate that the sensitivity of the test employed in the detection of HPV has previously masked its presence.
The case presented by Sotlar et al[28] is also of particular interest, given that it reported the findings of adjacent squamous metaplasia, dysplasia, and carcinoma in sequence, with HPV 16 identified in all three components and the surrounding non-tumour affected rectal mucosa. This mirrors the pre-neoplastic to neoplastic progression well documented in HPV driven anogenital cancers. Furthermore, they identified transcriptional activity of the HPV E6/7 oncogenes critical to HPV’s role in carcinogenesis. This may suggest that there are two possible pathways to the pathogenesis of colorectal SCC, HPV driven and non-HPV driven. However, while there is currently limited evidence surrounding HPV in rectal SCC, a clear association and a role in causation remains to be proven.
Patients with HIV have a higher incidence of HPV infection than the general population and additionally, HIV infection increases susceptibility to virally promoted cancers including Burkitt’s lymphoma (Epstein barr virus), Kaposi’s sarcoma (human herpes virus 8) and anogenital carcinoma (HPV). Consequently, it could be inferred that the cell mediated immune deficiency associated with HIV would predispose to rectal SCC. However, this is not borne out on review of the literature, with only two case reports of rectal SCC in the setting of HIV infection[29,42].
Another postulated aetiology, has arisen from the finding of squamous differentiation within colorectal adenomas. Williams et al[1] found this to be present in 3 of 750 adenomas, with a separate villous adenoma containing both invasive squamous and adenocarcinoma. Others have reported squamous metaplasia in adenomatous polyps[43-46] in addition to a further case of SCC in a villous adenoma[47]. These findings may again represent the squamous differentiation of a basal colonic cell, with changes inciting development of the adenoma also possibly leading to the metaplastic change.
Clinical presentation and diagnosis: The pattern of presentation for patients with rectal SCC is similar to those with adenocarcinoma of the rectum. The most frequently reported symptom is per rectal bleeding, followed less commonly by altered bowel habit (constipation, diarrhoea, tenesmus), pain and weight loss[48]. The duration of symptoms can be variable, but most patients report a symptom history of weeks to months[49,50].
Many presumed rectal SCCs are in fact an extension of an anal or gynaecological carcinoma, and consequently vigilance in diagnosis is important. Certain exclusion criteria stipulated by Williams et al[1] in 1979, remain relevant for a diagnosis of primary rectal SCC to be established: (1) metastasis to the rectum from SCC of another organ; (2) squamous-lined fistula tract involving the affected region of rectum; and (3) SCC of anal or gynaecological origin extending into the rectum.
With the above in mind, a detailed history and physical examination should be undertaken, with particular attention to the gynaecological system and anal canal. This often necessitates an examination under anaesthesia of both systems in addition to endoscopy.
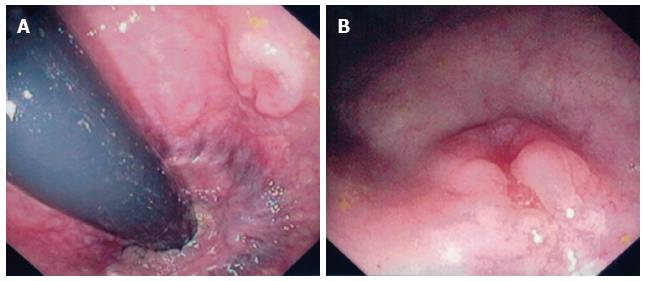
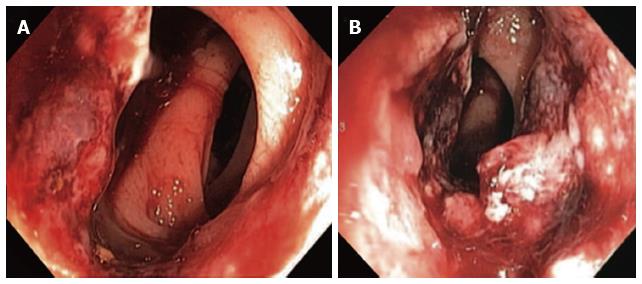
The definitive diagnosis of rectal SCC is confirmed by performing a complete colonoscopy with biopsies of any abnormalities. Demonstration of the discontinuity of a lesion from the anal squamous mucosa is of great importance. Rectal SCC has been reported to have a varied endoscopic appearance dependent on the stage of disease. This can range from a small mucosal polyp (Figure 4), plaque or ulceration through to a large obstructing mass (Figure 5)[51]. Pre-malignant lesions in the form of squamous metaplasia have also been identified by way of narrow band imaging (NBI) in addition to rectal SCC[32,52]. One report identified an appearance of dark brown dots similar to the intraepithelial papillary capillary loops (IPCL) which herald squamous epithelium in the oesophagus using NBI[32]. There are classification systems utilising the appearance of IPCL in the oesophagus in order to identify and differentiate squamous lesions along the spectrum towards invasive carcinoma[53]. Given the possible aetiological sequence of metaplasia through to invasive carcinoma, NBI may find a role in the detection and treatment of pre-malignant lesions for those at high risk, in particular ulcerative colitis patients.
Histologically, if the diagnosis remains unclear, immunohistochemistry can aid in the characterisation of the lesion. This is particularly useful in cases of poorly differentiated tumours where the morphology and architecture provide little clue to the origin. Cytokeratins AE1/AE3, CK 5/6 (34BE12 stains CK5) and p63 stain for cells of squamous origin, assisting in the differentiation from a rectal adenocarcinoma. Cytokeratin CAM5.2 aids in the differentiation of rectal from anal, characteristically staining for rectal squamous cell or adenocarcinoma but not anal SCC. This is particularly useful for squamous carcinomas of the lower rectum[16].
Squamous cell carcinoma associated antigen is a serum tumour marker expressed by epidermoid tumours, including squamous carcinomas of the anal canal. Despite studies demonstrating weak evidence it may relate to nodal or relapsed disease in anal SCC, its use in diagnosis and follow-up remains controversial[54-56]. With very limited data in the setting of rectal SCC, there is currently no clear utility for SCCAg in the diagnosis or management of these patients[57].
Staging: Accurate staging of rectal SCC is of critical importance, in the same way that it dictates prognosis and management in anal SCC and rectal adenocarcinoma. In the literature, various staging systems have been translated into use for rectal SCC, most commonly the tumour-node-metastasis (TNM) system for rectal adenocarcinoma[18,49,58-62] or the TNM system for anal SCC[16,63,64]. While arguments can be made for the use of either staging system, the AJCC staging for rectal carcinoma is likely to have the greatest relevance. Firstly, the tumour stage focuses on the importance of the level of invasion through the rectal wall rather than the maximal dimension of the carcinoma. Secondly, nodal involvement is likely to follow the lymphatic drainage to the mesorectum and higher echelons, in preference to the alternative routes often involved in anal carcinoma such as the inguinal basins.
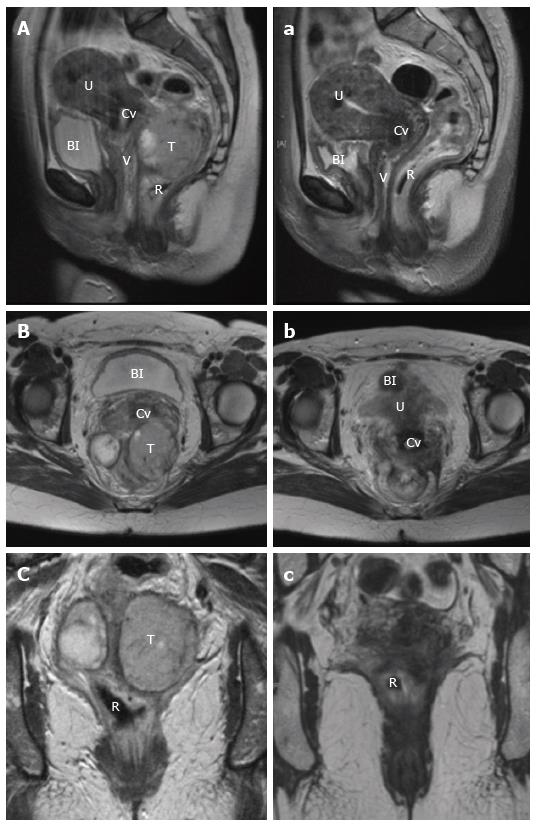
Staging involves evaluation of the primary tumour, and assessment for regional and metastatic disease. For loco-regional evaluation, as with rectal adenocarcinoma, magnetic resonance imaging (MRI) pelvis and endorectal ultrasound (ERUS) both have a role[65]. A preference for either modality is often dependent on the experience with each technique at individual institutions. In terms of utility, ERUS has advantage in determining the depth of tumour invasion, particularly with differentiating T1/2 lesions. For delineation of more advanced T3/4 tumours and to determine local nodal involvement, pelvic MRI provides improved definition[65,66]. Recently, there has been growing interest in the use of MRI diffusion weighted imaging (DWI) as a functional modality to assess treatment response in the staging of rectal adenocarcinoma[67]. With the current shift towards definitive chemoradiotherapy in the treatment of rectal SCC, MRI is likely to find an increasingly useful role, not only for structural pre-treatment staging, but more importantly to determine the functional response of the tumour post-treatment in order to guide the need for operative intervention (Figure 6).
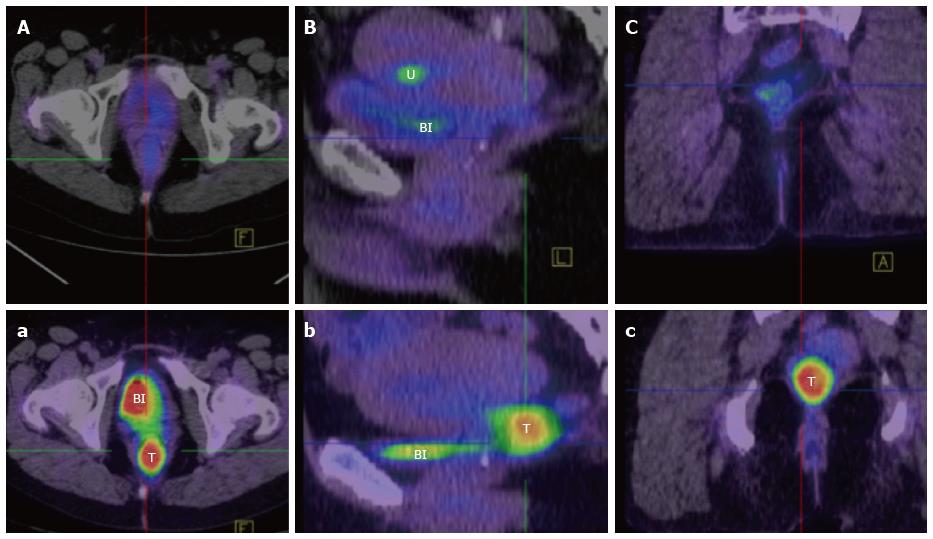
Computed tomography (CT) chest, abdomen and pelvis should be undertaken routinely in order to exclude metastatic disease. Increasingly, Fluorodeoxyglucose - positron emission tomography fused with simultaneous CT and more recently MRI imaging, is also finding a role in the staging of rectal SCC. Firstly, it allows exclusion of a non-rectal primary SCC that has metastasised to the rectum. Secondly it defines the extent of the primary and nodal disease. Thirdly, it has utility similar to MRI DWI imaging, in assessing the functional response of the tumour by comparing pre and post-treatment scans (Figure 7)[65,68].
The treatment of rectal SCC has traditionally involved surgery, in some cases preceded or followed by adjuvant radiotherapy or chemotherapy (Table 1)[16,69]. However, in the last decade, there has been increasing interest in the response of rectal SCC to definitive chemoradiotherapy, with very encouraging results (Table 2).
| Ref. | Pts | Initial Rx | Surgery | Adjuvant Rx | Recurrence | Survival (ANED) | Follow up (mo) |
| Raiford et al[5] | 1 | - | PP | - | 1 - LR | 0% | 21 |
| Catell et al[81] | 1 | - | APR | - | - | 100% (1) | 42 |
| LeBlanc et al[30] | 5 | - | 1 - APR, PR - 4 | - | 1 - LR | 40% (2) | 3-60 |
| O'Brien et al[82] | 2 | - | APR × 2 | - | - | 100% (2) | 12 |
| Kron et al[83] | 1 | - | APR | - | 1 - M, 1 - LR | 0% | 5 |
| 2Dixon et al[84] | 1 | - | PR | - | NR | NR | - |
| Burns et al[85] | 1 | - | APR | - | - | 100% (1) | 42 |
| Wiener et al[86] | 1 | - | APR | - | M - 1, LR - 1 | 0% | 12 |
| Zirkin et al[6] | 1 | - | TPC/APR | - | - | 100% (1) | 16 |
| Hohm et al[7] | 2 | - | APR × 2 | - | - | 100% (1) | 156-252 |
| Angelchik et al[87] | 1 | - | AR | - | - | 100% (1) | 18 |
| Cabrera et al[34] | 1 | - | APR | - | - | 100% (1) | 10 |
| Minkowitz et al[24] | 1 | - | TPC | - | M - 1 | 0% | 5 |
| 2Higton et al[11] | 1 | - | AR | - | NR | NR | - |
| Comer et al[10] | 1 | - | APR | - | - | 100% (1) | 156 |
| Williams et al[1] | 1 | - | APR | - | M - 1 | 0% | 9 |
| Vezeridis et al[25] | 4 | CTx - 1 | APR - 3 | CTx - 1 | M - 2, LR - 1 | 0% | 0-15 |
| Lafreniere et al[48] | 1 | - | TAE | CRTx | - | 100% (1) | 24 |
| Pigott et al[88] | 1 | - | APR | RTx | - | 100% (1) | 13 |
| Woods et al[35] | 1 | - | APR | - | - | 0% | 3 |
| Prener et al[71] | 5 | - | APR - 4, TAE - 1 | RTx - 1 | LR - 3, M - 1 | 20% (1) | 3-36 |
| Schneider et al[70] | 1 | - | TAE | CRTx | - | 100% (1) | 6 |
| Fazzi et al[89] | 1 | - | Y | RTx | - | 100% (1) | 72 |
| Copur et al[90] | 1 | - | APR | CRTx | M - 1 | NR | - |
| 2Frizelle et al[3] | 9 | - | NR | NR | NR | NR | - |
| Sotlar et al[28] | 1 | - | AR | - | LR - 1 | 0% | 21 |
| Gelas et al[69] | 4 | RTx - 2 | APR x 3 | CRTx - 1, RTx - 1 | LR - 1, M - 2 | 25% (1) | 4-192 |
| Anagnostopoulos et al[91] | 1 | - | APR | CTx | - | 100% (1) | 14 |
| Fahim et al[92] | 1 | - | APR | CTx | LR - 1, M - 1 | 0% | 11 |
| 2Lam et al[93] | 1 | RTx | AR | - | - | NR | - |
| 2Cheng et al[15] | 1 | - | TPC | CRTx | - | NR | - |
| Kong et al[12] | 2 | CTx - 1 | TAE - 1 | CRTx - 1 | - | 50% (1) | 36 |
| Nahas et al[16] | 3 | CTx - 1 | APR, TAE | CRTx - 2 | - | 100% (3) | 6-192 |
| 1Leung et al[20] | 1 | - | S | - | M - 1 | 0% | - |
| 2Dzeletovic et al[52] | 1 | NR | NR | NR | NR | NR | - |
| Sameer et al[94] | 1 | - | AR | CTx | - | 100% (1) | 24 |
| Wang et al[60] | 2 | - | H, TAE | CRTx - 2 | M - 1 | 50% (1) | 21, 120 |
| Sanal et al[95] | 1 | CTx | AR | - | - | 100% (1) | 12 |
| Yeh et al[49] | 1 | - | APR | M - 1 | 0% | 7 | |
| Faidzal et al[96] | 1 | - | AR | CRTx | - | 100% (1) | 15 |
| Wang et al[97] | 1 | - | APR | RTx | - | 100% (1) | 43 |
| Scaringi et al[23] | 1 | - | AR | - | LR - 1, M - 1 | 0% | 4 |
| Ozuner et al[14] | 7 | - | APR - 3 AR – 1 TPC - 1 TAE 1, H – 1 | CTx - 4 | M - 4, LR - 3 | 43% (3) | 12-96 |
| Péron et al[59] | 1 | RTx | - | - | LR - 1 | 0% | 40 |
| Overall | 63 (782) | CTx 4 RTx 4 | Resection 53 (PP/PR 6, APR 34, TPC/AR 13) TAE 7, H 2, S 1 | CRTx 11 CTx 8 RTx 5 | LR 25% (16) M 30% (19) | 48% (30) | 0-252 |
| Ref. | Pts | Chemotherapy | RTx (Gy) | CR | Surgery | Path CR | Recurrence | Survival(ANED) | Follow up(mo) | |
| 5FU/MMC | Other | |||||||||
| Vezeridis et al[25] | 1 | - | 1 | 40 | - | - | - | LR - 1 | 0% | 15 |
| 1Schneider et al[70] | 1 | 1 | - | 30 | - | - | - | - | NR | - |
| Kulayat et al[13] | 1 | 1 | - | 40 | - | TPC | 100% | - | 100% (1) | 48 |
| Martinez-Gonzalez et al[75] | 1 | - | 1 | 46 | - | AR | 0% | - | 100% (1) | 18 |
| Gelas et al[69] | 2 | - | 2 | Y | - | AR - 2 | 0% | - | 100 % (2) | 6-24 |
| Theodosopoulos et al[98] | 1 | 1 | - | 20 | - | APR | 0% | M - 1 | 100% (1) | 18 |
| Pikarsky et al[9] | 1 | 1 | - | 60 | 1 | - | - | - | 100% (1) | 84 |
| Nahas et al[16] | 9 | 6 | 3 | 50.4 | 2 | TAE - 2 APR - 2 AR - 3 | 86% | - | 100% (9) | 6-192 |
| Clark et al[61] | 7 | 3 | 4 | 50.4 | 7 | AR - 1 | 100% | - | 100% (7) | 5-31 |
| Matsuda et al[29] | 1 | - | 1 | 59.4 | - | APR | 0% | LR - 1, M - 1 | 0% | 24 |
| Brammer et al[99] | 1 | 1 | - | Y | 1 | - | - | M - 1 | 100% (1) | 24 |
| Rasheed et al[57] | 6 | 2 | 4 | 45-50.4 | 4 | APR - 2 | 50% | LR - 1 | 100% (6) | 2-132 |
| Al Hallak et al[100] | 1 | 1 | - | Y | 1 | - | - | - | 100% (1) | 30 |
| Tronconi et al[58] | 6 | 1 | 5 | 50.4-59.4 | 4 | AR - 1 H - 1 | 50% | M - 1 | 83% (5) | 24-41 |
| Iannacone et al[101] | 1 | 1 | - | 59.4 | 1 | - | - | - | 100% (1) | 12 |
| Wang et al[60] | 5 | 5 | - | 45-54 | 4 | AR - 2 APR - 1 | 100% | M - 2 | 60% (3) | 15-51 |
| Yeh et al[49] | 5 | 4 | 1 | 30-60 | 4 | AR - 1 | 100% | LR + M - 1 | 80% (4) | 24-84 |
| Jeong et al[62] | 4 | - | 4 | 50.4-63 | 4 | - | - | - | 75% (3) | 2-99 |
| Kassir et al[102] | 1 | - | 1 | Y | - | AR | 0% | - | 100% (1) | - |
| Ferreira et al[64] | 1 | 1 | - | 52 | 1 | - | - | - | 100% (1) | 40 |
| Choi et al[42] | 1 | 1 | - | Y | 1 | APR | 0% | LR - 1 | 100% (1) | 17 |
| Musio et al[63] | 8 | 6 | 2 | 45-70.6 | 4 | APR - 4 | 50% | LR - 1 | 88% ( 7) | 1-164 |
| Péron et al[59] | 10 | 4 | 6 | 45-62 | 6 | APR - 2 AR - 2 | 50% | LR - 1 | 100% (10) | 6-133 |
| Funahashi et al[76] | 3 | - | 3 | 45-59.4 | 2 | PE | 100% | M - 1 | 67% (2) | 14-44 |
| Seshadri et al[103] | 1 | 1 | - | 50.4 | 1 | AR | 0% | LR + M - 1 | 0% | 36 |
| Ozuner et al[14] | 1 | 1 | - | Y | - | - | 0% | M - 1 | 0% | 12-96 |
| Overall | 79 (801) | 42 | 38 | All | 60% (48) | 44% (35) | 57% (20) | LR 10% (8) M 13% (10) | 86% (68) | 1-192 |
Surgery: Surgery has historically been adopted from the treatment of rectal adenocarcinoma with the operative technique, dependent upon the stage and location of the tumour. Local excision either trans-anal or endoscopic, has been advocated for selected cases, with several publications reporting short-term survival without recurrence in the setting of trans-anal excision followed by chemoradiotherapy[12,16,48,70,71]. This included a T3 lesion and another with positive distal and radial margins, suggesting the chemoradiation may have played an important role in reducing local recurrence[16,70]. With the evolution of endoscopic techniques, in particular endoscopic mucosal resection and submucosal dissection in the treatment of early rectal cancers[72], these procedures may have a role in managing rectal SCC. Generally, the option of local excision would be limited to low risk T1 lesions, characterised as being well differentiated, without lymphovascular involvement, nodal or metastatic disease.
For most rectal SCC’s, anterior resection (AR) or abdominoperineal resection (APR) has classically been performed. The choice and extent of the operation is dependent upon the tumour location and depth of invasion, occasionally requiring exenteration, with removal of involved pelvic structures. On review of the literature, APR was performed much more frequently than AR prior to the year 2000, with an equal split in the frequency of both procedures following the turn of the century (Table 1). This is likely to reflect both the change towards sphincter preservation and avoidance of a permanent stoma in operations for rectal cancer over previous decades, in addition to a down-staging effect of chemoradiation, which is now commonplace. While the incidence of APR and a definitive stoma has been falling, in a similar manner to rectal adenocarcinoma, most patients with a low rectal SCC will require a temporary covering ileostomy given the greater risk of anastomotic leak. Furthermore, for those patients presenting with an obstructing tumour, the use of a defunctioning stoma is an attractive option, providing time to appropriately stage the patient and consider the optimal treatment, including definitive chemoradiotherapy.
Chemoradiotherapy: Following the validation of Nigro’s protocol in multiple randomised controlled trials, it has now become the accepted standard treatment for anal SCC. Surgery, previously the preferred management, has subsequently been relegated to a salvage role[73,74]. In light of this development, a trend of treating rectal SCC in the same manner has emerged.
On review of the literature, which spans from 1933 to the present, it is difficult to compare the treatment of rectal SCC, given the lack of a standardised staging system and treatment protocol. Nonetheless, an increasing trend in the use of chemoradiation either as definitive treatment or in conjunction with surgery is emerging. There have been several prospective studies evaluating the role of chemoradiation as the primary therapy. The earlier cohorts demonstrated suboptimal outcomes, without a change in mortality or avoidance of surgery[25,75]. However, with improvements in chemotherapy, radiotherapy and the accuracy of determining stage and response, a multitude of recent studies utilising an anal SCC based treatment regimen have reported promising results (Table 2)[3,16,49,57,61,63,69].
The 3 most recent case series all published in 2015, comprise 22 patients treated with definitive chemoradiotherapy. Of this grouped cohort, a CR was identified by clinical examination and/or imaging in 14 of the 22 patients[59,63,76]. The remaining 8 patients who demonstrated either progression of disease, a partial response or discordance between clinical and radiological findings, underwent a salvage operation. Of this group, 5 were noted to have a complete pathological response, equating to 19 of the 22 patients demonstrating a CR. Median follow up was 25 mo, with three patients suffering a recurrence, two of whom underwent a salvage operation, and one who received radiotherapy given the recurrence was outside the original field of treatment. Of these three, one succumbed to their disease at 14 mo post salvage surgery. One patient with an initial partial response and subsequent salvage operation developed metastatic disease without local relapse. The remaining 20 patients are alive without evidence of disease[59,63,76].
Expanding from the above findings, when all cases reported in the literature are examined, it is obvious that patients undergoing definitive chemoradiotherapy have a far superior survival then what has been historically recorded (Table 1 compared with Table 2). The overall survival for the chemoradiation group was 86% compared with 48% for conventional treatment. Likewise, the local recurrence and metastatic rates were respectively 25% vs 10% and 30% vs 13% for the chemoradiation vs conventional treatment cohorts. These differences are likely due to a combination of factors, including improvements in imaging, tumour staging and perioperative workup and patient care over time. Furthermore, there are significant limitations in the analysis and interpretation of these results, related to the inherent heterogeneity of case reports and the inconsistency in recording important prognostic variables including stage and grade. Despite these limitations, the treatment itself almost certainly accounts for a significant component of the dramatically improved local control and survival.
As with rectal and anal cancer, one of the most pertinent issues with definitive chemoradiation, is determining treatment response, which currently can only be confirmed on histopathology[73,77]. In the studies to date on rectal SCC, response to chemoradiotherapy has been assessed variably, from 6-8 wk up to 6 mo after the conclusion of treatment. This generally involves a combination of a clinical assessment, by way of a repeat EUA/proctoscopy + biopsy, and an imaging assessment in the form of MRI ± PET/CT ± ERUS[49,58,60,61]. For patients with a complete clinical and radiological response, follow up and surveillance is performed at regular intervals with reducing frequency out to five years, generally 3 monthly for the first two years, and 6 monthly out to five years. While this is certainly labour and resource intensive with consequent costs, the improved overall and stoma free survival certainly justifies this approach.
For those cases with clear progression of disease through chemoradiation, salvage surgery should be undertaken as the next line of treatment to ensure optimal outcomes. However, in the setting of a partial response or stable disease, the pathway is less clear. It has been suggested that a more prolonged assessment, with regular EUAs even out to 6 mo, could be required for a better evaluation of tumour response. This is in consideration of the finding that multiple patients with an eventual pathological CR had clinical and radiological findings suggestive of persistent disease in the early post chemoradiation stage[16]. This is also in keeping with the accepted management of anal SCC, where a delayed tumour response may continue for 6 mo after the completion of chemoradiation[63]. In the grouped chemoradiation cohort (Table 2), a CR on pathology was identified in 57% of patients, suggesting that time may have played a role in assessing clinical and radiological response. As with rectal and anal cancer, this is likely to remain a contentious area until a more effective means of determining response is available[78].
Despite the encouraging results of chemoradiotherapy, currently a set treatment protocol is yet to be established. It appears that 5FU based chemotherapy combined with high dose external beam radiotherapy may be efficacious. However, while these trends are grossly evident from the literature, there is a need for further research in order to determine the most effective regimen to optimise patient outcomes.
It is unlikely that a randomised trial comparing surgery and chemoradiotherapy will ever be conducted for this rare cancer. Given the current knowledge base, it may be reasonable to suggest that primary treatment should be chemoradiotherapy, with surgery reserved as a salvage option. The suggested regimen would be a total dose of 50.4 to 54 Gy external beam radiation in 1.8 Gy per fraction, given concurrently with 5FU and mitomycin C.
Future options: Over recent years, there has been an increasing use of molecular targeted therapies in solid and haematological malignancies[79]. Furthermore, immunotherapy in the form of tumour vaccines, adoptive T cell therapy and immune checkpoint inhibitors has become a major focus for research in the treatment of cancer, with translated clinical success in specific tumour types[80]. While there is currently no literature on these modalities in rectal SCC, the early results in other tumours holds promise for a possible role in future treatment, particularly in the cohort of patients with persistent, recurrent or metastatic rectal SCC.
The most important predictor of survival in all cancers is the stage of disease. This is based upon three factors; the size of the primary tumour and depth of invasion (T stage); the location and number of lymph nodes involved (N stage); and the presence or absence of metastasis (M stage). Rectal SCC follows the same route of lymphatic spread for involvement of lymph nodes as rectal adenocarcinoma. Additionally, it has a similar pattern of metastasis with the liver, lung and bones most commonly affected[66].
While the majority of patients with rectal SCC present with locoregional disease (stage I-III, 82.1%), they are associated with a poorer overall survival when compared stage for stage with adenocarcinoma. From review of the population study by the NCI, the overall 5-year survival for rectal SCC was found to be 48.9% compared with 62.1% for adenocarcinoma. When localised, the 5 years OS was 73.7% (91.8% - adenocarcinoma), with 31.3% (65.8%) for regional and 20.8% (8.8%) for metastatic[2]. While the above figures and those from older studies report a poor prognosis for patients with rectal SCC, recent studies employing a new treatment paradigm, demonstrate a significantly improved overall survival. The possibility of further advances in treatment as this disease is better defined, gives hope for improved patient outcomes.
While SCC of the rectum is a rare entity, there is an increasing body of evidence that is improving our understanding of its underlying aetiology. Despite the literature lacking uniformity in the staging and management of rectal SCC, it is hard to ignore the impressive improvements in overall survival and sphincter preservation by way of chemoradiotherapy as the primary modality of treatment. This holds much promise for the future, and certainly lays the foundation for further investigation into determining the optimal treatment regimen.
A summary of the current body of knowledge surrounding the pathogenesis, presentation, diagnosis, staging and management of rectal squamous cell carcinoma (SCC), with a focus on the changing treatment paradigm and consequent improved patient outcomes.
While the underlying pathogenesis of rectal SCC is yet to be fully defined, a possible role for human papilloma virus presents an avenue for future investigation. Furthermore, the identification of pre-malignant lesions in the development of rectal SCC raises the possibility of surveillance in high risk patients. The use of magnetic resonance imaging (MRI) and positron emission tomography (PET) has an emerging role not only in diagnosis and staging, but also importantly as a functional modality to determine response to chemoradiotherapy. This role has arisen from the recent shift in the primary treatment of rectal SCC to chemoradiotherapy, accompanied by a dramatic improvement in overall survival.
Assimilation of the current body of evidence lends support to the presence of pre-malignant lesions and a metaplasia-dysplasia-carcinoma sequence in the development of rectal SCC. Staging for rectal SCC fits more appropriately with the tumour-node-metastasis (TNM) criteria for rectal adenocarcinoma than anal SCC. MRI and PET are finding an increasing role in diagnosis, staging and assessment of response to treatment in rectal SCC. Chemoradiotherapy offers improved patient outcomes without the associated morbidity of surgery. Improved markers of complete response will assist in determining the need for salvage treatment in this patient cohort.
Consideration should be given to screening for premalignant lesions in high risk individuals. Uniform staging utilising the current TNM criteria for rectal adenocarcinoma should be encouraged. PET and MRI should be incorporated into the evaluation of patients, pre and post treatment. Definitive chemoradiotherapy offers improved patient outcomes without the associated morbidity of surgery. While treatment must be individualised and based on patient and tumour factors, chemoradiation should form the basis of primary management.
Complete response refers to the resolution of tumour following treatment with chemoradiotherapy. While radiological investigations and clinical examination can act as surrogate markers, a true complete response can currently only be determined post resection and pathological examination.
The review article described the background, diagnosis and staging, treatment, and prognosis of primary SCC of the rectum. The whole article is well written and characterized.
P- Reviewer: Bao Y, Butterworth J, De Nardi P, El-Tawil AM, Schofield JB, Suzuki N S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol. 1979;129:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Kang H, O’Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Frizelle FA, Hobday KS, Batts KP, Nelson H. Adenosquamous and squamous carcinoma of the colon and upper rectum: a clinical and histopathologic study. Dis Colon Rectum. 2001;44:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Schmidtmann M. Zur Kenntnis seltener Krebsformen. Virchows Arch Pathol Anat. 1919;226:100-118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Raiford T. Epitheliomata of the lower rectum and anus. Surg Gynaecol Obstet. 1933;57:21-35. |
| 6. | Zirkin RM, Mccord DL. Squamous cell carcinoma of the rectum: report of a case complicating chronic ulcerative colitis. Dis Colon Rectum. 1963;6:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Hohm WH, Jackman RJ. Squamous cell carcinoma of the rectum complicating ulcerative colitis: report of two cases. Mayo Clin Proc. 1964;39:249-251. [PubMed] |
| 8. | Michelassi F, Montag AG, Block GE. Adenosquamous-cell carcinoma in ulcerative colitis. Report of a case. Dis Colon Rectum. 1988;31:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Pikarsky AJ, Belin B, Efron J, Woodhouse S, Weiss EG, Wexner SD, Nogueras JJ. Squamous cell carcinoma of the rectum in ulcerative colitis: case report and review of the literature. Int J Colorectal Dis. 2007;22:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Comer TP, Beahrs OH, Dockerty MB. Primary squamous cell carcinoma and adenocanthoma of the colon. Cancer. 1971;28:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Higton DI. Squamous cell carcinoma of rectum. Proc R Soc Med. 1970;63:754. [PubMed] |
| 12. | Kong CS, Welton ML, Longacre TA. Role of human papillomavirus in squamous cell metaplasia-dysplasia-carcinoma of the rectum. Am J Surg Pathol. 2007;31:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kulaylat MN, Doerr R, Butler B, Satchidanand SK, Singh A. Squamous cell carcinoma complicating idiopathic inflammatory bowel disease. J Surg Oncol. 1995;59:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ozuner G, Aytac E, Gorgun E, Bennett A. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis. 2015;30:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Cheng H, Sitrin MD, Satchidanand SK, Novak JM. Colonic squamous cell carcinoma in ulcerative colitis: Report of a case and review of the literature. Can J Gastroenterol. 2007;21:47-50. [PubMed] |
| 16. | Nahas CS, Shia J, Joseph R, Schrag D, Minsky BD, Weiser MR, Guillem JG, Paty PB, Klimstra DS, Tang LH. Squamous-cell carcinoma of the rectum: a rare but curable tumor. Dis Colon Rectum. 2007;50:1393-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Jaworski RC, Biankin SA, Baird PJ. Squamous cell carcinoma in situ arising in inflammatory cloacogenic polyps: report of two cases with PCR analysis for HPV DNA. Pathology. 2001;33:312-314. [PubMed] |
| 18. | Audeau A, Han HW, Johnston MJ, Whitehead MW, Frizelle FA. Does human papilloma virus have a role in squamous cell carcinoma of the colon and upper rectum. Eur J Surg Oncol. 2002;28:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Pittella JE, Torres AV. Primary squamous-cell carcinoma of the cecum and ascending colon: report of a case and review of the literature. Dis Colon Rectum. 1982;25:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Leung KK, Heitzman J, Madan A. Squamous cell carcinoma of the rectum 21 years after radiotherapy for cervical carcinoma. Saudi J Gastroenterol. 2009;15:196-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Yurdakul G, de Reijke TM, Blank LE, Rauws EA. Rectal squamous cell carcinoma 11 years after brachytherapy for carcinoma of the prostate. J Urol. 2003;169:280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 22. | Pemberton M, Lendrum J. Squamous-cell carcinoma of the caecum following ovarian adenocarcinoma. Br J Surg. 1968;55:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Scaringi S, Bisogni D, Messerini L, Bechi P. Squamous cell carcinoma of the middle rectum: Report of a case and literature overview. Int J Surg Case Rep. 2015;7C:127-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Minkowitz S. Primary squamous cell carcinoma of the rectosigmoid portion of the colon. Arch Pathol. 1967;84:77-80. [PubMed] |
| 25. | Vezeridis MP, Herrera LO, Lopez GE, Ledesma EJ, Mittleman A. Squamous-cell carcinoma of the colon and rectum. Dis Colon Rectum. 1983;26:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Petrelli NJ, Valle AA, Weber TK, Rodriguez-Bigas M. Adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum. 1996;39:1265-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Birnbaum W. Squamous cell carcinoma and adenoacanthoma of the colon. JAMA. 1970;212:1511-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Sotlar K, Köveker G, Aepinus C, Selinka HC, Kandolf R, Bültmann B. Human papillomavirus type 16-associated primary squamous cell carcinoma of the rectum. Gastroenterology. 2001;120:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Matsuda A, Takahashi K, Yamaguchi T, Matsumoto H, Miyamoto H, Kawakami M, Kawachi H, Suzuki H, Furukawa K, Tajiri T. HPV infection in an HIV-positive patient with primary squamous cell carcinoma of rectum. Int J Clin Oncol. 2009;14:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | LeBlanc LJ, Buie LA, Dockerty MB. Squamous-cell epithelioma of the rectum. Ann Surg. 1950;131:392-399. [PubMed] |
| 31. | Lugo M, Putong PB. Metaplasia. An overview. Arch Pathol Lab Med. 1984;108:185-189. [PubMed] |
| 32. | Fu K, Tsujinaka Y, Hamahata Y, Matsuo K, Tsutsumi O. Squamous metaplasia of the rectum associated with ulcerative colitis diagnosed using narrow-band imaging. Endoscopy. 2008;40 Suppl 2:E45-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Lee SD, Haggitt RC, Kimmey MB. Squamous metaplasia of the rectum after argon plasma coagulation. Gastrointest Endosc. 2000;52:683-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Cabrera A, Pickren JW. Squamous metaplasia and squamous-cell carcinoma of the rectosigmoid. Dis Colon Rectum. 1967;10:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Woods WG. Squamous cell carcinoma of the rectum arising in an area of squamous metaplasia. Eur J Surg Oncol. 1987;13:455-458. [PubMed] |
| 36. | Dukes CE. The Surgical Significance of the Unusual in the Pathology of Intestinal Tumours: Imperial Cancer Research Fund Lecture delivered at the Royal College of Surgeons of England on 23rd November, 1948. Ann R Coll Surg Engl. 1949;4:90-103. |
| 37. | Reeve DR. Squamous metaplasia in the healing of chronic colonic ulcers of the rat. J Pathol. 1975;117:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Wells HG, Slye M, Holmes HF. Comparative Pathology of Cancer of the Alimentary Canal, with Report of Cases in Mice Studies in the Incidence and Inheritability of Spontaneous Tumors in Mice: 34th Report. Am J Cancer. 1938;33:223-238. [DOI] [Full Text] |
| 39. | Hicks JD, Cowling DC. Squamous-cell carcinoma of the ascending colon. J Pathol Bacteriol. 1955;70:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Ouban A, Nawab RA, Coppola D. Diagnostic and pathogenetic implications of colorectal carcinomas with multidirectional differentiation: a report of 4 cases. Clin Colorectal Cancer. 2002;1:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Michelassi F, Mishlove LA, Stipa F, Block GE. Squamous-cell carcinoma of the colon. Experience at the University of Chicago, review of the literature, report of two cases. Dis Colon Rectum. 1988;31:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Choi H, Lee HW, Ann HW, Kim JK, Kang HP, Kim SW, Ku NS, Han SH, Kim JM, Choi JY. A Case of Rectal Squamous Cell Carcinoma with Metachronous Diffuse Large B Cell Lymphoma in an HIV-Infected Patient. Infect Chemother. 2014;46:257-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Chen KT. Colonic adenomatous polyp with focal squamous metaplasia. Hum Pathol. 1981;12:848-849. [PubMed] |
| 44. | Almagro UA, Pintar K, Zellmer RB. Squamous metaplasia in colorectal polyps. Cancer. 1984;53:2679-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Kontozoglou T. Squamous metaplasia in colonic adenomata: report of two cases. J Surg Oncol. 1985;29:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Forouhar F. Neoplastic colonic polyp with extensive squamous metaplasia. Case report. Tumori. 1984;70:99-103. [PubMed] |
| 47. | Lundquest DE, Marcus JN, Thorson AG, Massop D. Primary squamous cell carcinoma of the colon arising in a villous adenoma. Hum Pathol. 1988;19:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Lafreniere R, Ketcham AS. Primary squamous carcinoma of the rectum. Report of a case and review of the literature. Dis Colon Rectum. 1985;28:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Yeh J, Hastings J, Rao A, Abbas MA. Squamous cell carcinoma of the rectum: a single institution experience. Tech Coloproctol. 2012;16:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol. 2009;15:4380-4386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Errasti Alustiza J, Espín Basany E, Reina Duarte A. Rare tumors of the rectum. Narrative review. Cir Esp. 2014;92:579-588. [PubMed] |
| 52. | Dzeletovic I, Pasha S, Leighton JA. Human papillomavirus-related rectal squamous cell carcinoma in a patient with ulcerative colitis diagnosed on narrow-band imaging. Clin Gastroenterol Hepatol. 2010;8:e47-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Boeriu A, Boeriu C, Drasovean S, Pascarenco O, Mocan S, Stoian M, Dobru D. Narrow-band imaging with magnifying endoscopy for the evaluation of gastrointestinal lesions. World J Gastrointest Endosc. 2015;7:110-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Petrelli NJ, Palmer M, Herrera L, Bhargava A. The utility of squamous cell carcinoma antigen for the follow-up of patients with squamous cell carcinoma of the anal canal. Cancer. 1992;70:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 55. | Fontana X, Lagrange JL, Francois E, Bourry J, Chauvel P, Sordage M, Lapalus F, Namer M. Assessment of “squamous cell carcinoma antigen” (SCC) as a marker of epidermoid carcinoma of the anal canal. Dis Colon Rectum. 1991;34:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Indinnimeo M, Reale MG, Cicchini C, Stazi A, Fiori E, Izzo P. CEA, TPA, CA 19-9, SCC and CYFRA at diagnosis and in the follow-up of anal canal tumors. Int Surg. 1997;82:275-279. [PubMed] |
| 57. | Rasheed S, Yap T, Zia A, McDonald PJ, Glynne-Jones R. Chemo-radiotherapy: an alternative to surgery for squamous cell carcinoma of the rectum--report of six patients and literature review. Colorectal Dis. 2009;11:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Tronconi MC, Carnaghi C, Bignardi M, Doci R, Rimassa L, Di Rocco M, Scorsetti M, Santoro A. Rectal squamous cell carcinoma treated with chemoradiotherapy: report of six cases. Int J Colorectal Dis. 2010;25:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Péron J, Bylicki O, Laude C, Martel-Lafay I, Carrie C, Racadot S. Nonoperative management of squamous-cell carcinoma of the rectum. Dis Colon Rectum. 2015;58:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Wang ML, Heriot A, Leong T, Ngan SY. Chemoradiotherapy in the management of primary squamous-cell carcinoma of the rectum. Colorectal Dis. 2011;13:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Clark J, Cleator S, Goldin R, Lowdell C, Darzi A, Ziprin P. Treatment of primary rectal squamous cell carcinoma by primary chemoradiotherapy: should surgery still be considered a standard of care. Eur J Cancer. 2008;44:2340-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Jeong BG, Kim DY, Kim SY. Concurrent chemoradiotherapy for squamous cell carcinoma of the rectum. Hepatogastroenterology. 2013;60:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 63. | Musio D, De Felice F, Manfrida S, Balducci M, Meldolesi E, Gravina GL, Tombolini V, Valentini V. Squamous cell carcinoma of the rectum: The treatment paradigm. Eur J Surg Oncol. 2015;41:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Ferreira AO, Loureiro AL, Marques V, Sousa HT. Primary squamous cell carcinoma of the most distal rectum: a dilemma in origin and management. BMJ Case Rep. 2014;2014:pii: bcr2013201156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Greene FL, Hindman NM, Jones B, Katz DS. ACR Appropriateness Criteria pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012;9:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Wu JS. Rectal cancer staging. Clin Colon Rectal Surg. 2007;20:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Hötker AM, Garcia-Aguilar J, Gollub MJ. Multiparametric MRI of rectal cancer in the assessment of response to therapy: a systematic review. Dis Colon Rectum. 2014;57:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Agarwal A, Marcus C, Xiao J, Nene P, Kachnic LA, Subramaniam RM. FDG PET/CT in the management of colorectal and anal cancers. AJR Am J Roentgenol. 2014;203:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Gelas T, Peyrat P, Francois Y, Gerard JP, Baulieux J, Gilly FN, Vignal J, Glehen O. Primary squamous-cell carcinoma of the rectum: report of six cases and review of the literature. Dis Colon Rectum. 2002;45:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Schneider TA, Birkett DH, Vernava AM. Primary adenosquamous and squamous cell carcinoma of the colon and rectum. Int J Colorectal Dis. 1992;7:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Prener A, Nielsen K. Primary squamous cell carcinoma of the rectum in Denmark. APMIS. 1988;96:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Baig KRKK, Wallace MB. Endoscopic Mucosal Resection: Therapy for Early Colorectal Cancer. J Cancer Ther. 2013;4:8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, Arnold D. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol. 2014;111:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 74. | Nigro ND. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis Colon Rectum. 1984;27:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 171] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Martinez-Gonzalez MD, Takahashi T, Leon-Rodriguez E, Gamboa-Dominguez A, Lome C, Garcia-Blanco MC, Bezaury P, Moran MA. Case report of primary squamous carcinoma of the rectum. Rev Invest Clin. 1996;48:453-456. [PubMed] |
| 76. | Funahashi K, Nemoto T, Koike J, Kurihara A, Shiokawa H, Ushigome M, Kaneko T, Arai K, Nagashima Y, Koda T. Chemoradiation therapy with S-1 for primary squamous cell carcinoma of the rectum: report of three cases. Surgical Case Reports. 2015;1:1-7. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Walker AS, Zwintscher NP, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, Avital I, Brücher BL, Steele SR. Future directions for monitoring treatment response in colorectal cancer. J Cancer. 2014;5:44-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Benson AB, Arnoletti JP, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Dilawari RA, Engstrom PF, Enzinger PC. Anal Carcinoma, Version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:449-454. [PubMed] |
| 79. | Sawyers C. Targeted cancer therapy. Nature. 2004;432:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 806] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 80. | Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2878] [Article Influence: 205.6] [Reference Citation Analysis (0)] |
| 81. | Catell RB, Williams , AG . Epidermoid carcinoma of the anus and rectum. Arch Surg. 1943;46:336-349. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | O’brien JP, Meehan DJ. Squamous cell carcinoma of the rectum. Ann Surg. 1951;133:283-285. [PubMed] |
| 83. | Kron SD, Wurzel HA, Chodoff RJ. Squamous cell carcinoma of the rectum. Gastroenterology. 1951;17:194-197. [PubMed] |
| 84. | Dixon CF, Dockerty MB, Powelson MH. Squamous cell carcinoma of the midrectum: report of case. Proc Staff Meet Mayo Clin. 1954;29:420-423. [PubMed] |
| 86. | Wiener MF, Polayes SH, Yidi R. Squamous carcinoma with schistosomiasis of the colon. Am J Gastroenterol. 1962;37:48-54. [PubMed] |
| 87. | Angelchik JP, Epstein J. Squamous cell carcinoma of the upper rectum. Ariz Med. 1967;24:19-21. [PubMed] |
| 88. | Pigott JP, Williams GB. Primary squamous cell carcinoma of the colorectum: case report and literature review of a rare entity. J Surg Oncol. 1987;35:117-119. [PubMed] |
| 89. | Fazzi U, Anderson JR. Squamous carcinoma of the rectum. Br J Clin Pract. 1994;48:106-107. [PubMed] |
| 90. | Copur S, Ledakis P, Novinski D, Mleczko KL, Frankforter S, Bolton M, Fruehling RM, VanWie E, Norvell M, Muhvic J. Squamous cell carcinoma of the colon with an elevated serum squamous cell carcinoma antigen responding to combination chemotherapy. Clin Colorectal Cancer. 2001;1:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Anagnostopoulos G, Sakorafas GH, Kostopoulos P, Grigoriadis K, Pavlakis G, Margantinis G, Vugiouklakis D, Arvanitidis D. Squamous cell carcinoma of the rectum: a case report and review of the literature. Eur J Cancer Care (Engl). 2005;14:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Fahim F, Al-Salamah SM, Alam MK, Al-Akeely MH. Squamous cell carcinoma of colon and rectum. Saudi Med J. 2006;27:874-877. [PubMed] |
| 93. | Lam AK, Ho YH. Primary squamous cell carcinoma of the rectum in a patient on immunosuppressive therapy. Pathology. 2006;38:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Sameer AS, Syeed N, Chowdri NA, Parray FQ, Siddiqi MA. Squamous cell carcinoma of rectum presenting in a man: a case report. J Med Case Rep. 2010;4:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Sanal SM, Sivrikoz ON, Karapolat I, Karademir S. Complete clinical response in squamous cell carcinoma of the rectum with liver metastases. J Clin Oncol. 2011;29:e806-e808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 96. | Faidzal O, Azmi MN, Kalavathi R. Primary Squamous Cell Carcinoma of the Rectum: a Case Report. IMJM. 2013;12:87. |
| 97. | Wang JF, Wang ZX, Xu XX, Wang C, Liu JZ. Primary rectal squamous cell carcinoma treated with surgery and radiotherapy. World J Gastroenterol. 2014;20:4106-4109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Theodosopoulos TK, Marinis AD, Dafnios NA, Vassiliou JG, Samanides LD, Carvounis EE, Smyrniotis VE. Aggressive treatment of metastatic squamous cell carcinoma of the rectum to the liver: a case report and a brief review of the literature. World J Surg Oncol. 2006;4:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Brammer RD, Taniere P, Radley S. Metachronous squamous-cell carcinoma of the colon and treatment of rectal squamous carcinoma with chemoradiotherapy. Colorectal Dis. 2009;11:219-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Al Hallak MN, Hage-Nassar G, Mouchli A. Primary Submucosal Squamous Cell Carcinoma of the Rectum Diagnosed by Endoscopic Ultrasound: Case Report and Literature Review. Case Rep Gastroenterol. 2010;4:243-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Iannacone E, Dionisi F, Musio D, Caiazzo R, Raffetto N, Banelli E. Chemoradiation as definitive treatment for primary squamous cell cancer of the rectum. World J Radiol. 2010;2:329-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Kassir R, Baccot S, Bouarioua N, Petcu CA, Dubois J, Boueil-Bourlier A, Patoir A, Epin A, Ripamonti B, Tiffet O. Squamous cell carcinoma of middle rectum: Literature review. Int J Surg Case Rep. 2014;5:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Seshadri RA, Pancholi M, Jayanand SB, Chandrasekar S. Squamous cell carcinoma of the rectum: Is chemoradiation sufficient. J Cancer Res Ther. 2015;11:664. [PubMed] |