Published online Dec 27, 2016. doi: 10.4240/wjgs.v8.i12.792
Peer-review started: August 5, 2016
First decision: August 26, 2016
Revised: September 7, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 27, 2016
Processing time: 138 Days and 0 Hours
We report a case of severe, refractory gastrointestinal (GI) bleeding in a patient with hereditary hemorrhagic telangiectasia (HHT) whose massive transfusion dependence was lifted shortly after treatment with bevacizumab, an anti-vascular endothelial growth factor. The patient’s bleeding had been refractory to repeated endoscopic interventions, tranexamic acid, and tamoxifen. However, following treatment with bevacizumab at 5 mg/kg every other week, nearly 300 units of packed red blood cell transfusions were avoided in one year’s time. Despite its relatively high cost, bevacizumab may have a more active role in the management of severe GI bleeding in HHT if such remarkable response can be consistently demonstrated.
Core tip: Management of gastrointestinal (GI) bleeding in patients with hereditary hemorrhagic telangiectasia (HHT) can be challenging when the vascular lesions recur despite repeated endoscopic treatments. There is increasing evidence supporting the use of anti-angiogenesis agents in the management of bleeding in HHT patients. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has been shown to reduce recurrent epistaxis. This case demonstrates the therapeutic potential of bevacizumab in patients with severe GI bleeding requiring massive transfusions.
- Citation: Ou G, Galorport C, Enns R. Bevacizumab and gastrointestinal bleeding in hereditary hemorrhagic telangiectasia. World J Gastrointest Surg 2016; 8(12): 792-795
- URL: https://www.wjgnet.com/1948-9366/full/v8/i12/792.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i12.792
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant condition characterized by vascular malformations that occur systemically, leading to manifestations such as recurrent epistaxis, gastrointestinal (GI) bleeding, and arteriovenous malformations (AVM) of the lung, liver, and brain. Since the discovery of elevated vascular endothelial growth factor (VEGF) expression in patients with HHT[1], reports of bevacizumab, a monoclonal antibody against VEGF, in managing complications of HHT have emerged[2-16], with most of the reported experience in recurrent epistaxis. We present a case of HHT with massive, refractory transfusion requirements secondary to severe GI bleeding that did not respond to conventional therapy, but subsequently achieved transfusion-independence with anti-VEGF mono-therapy.
A 58-year-old female with HHT and positive family history, involving her mother, sister, and daughter, presents with a 30-plus-year history of increasing transfusion dependency due to a combination of daily epistaxis, gastrointestinal bleeding, and intermittent gross hematuria. She did not have hemoptysis. Relevant medical history included hepatitis C infection secondary to transfusions, pulmonary hypertension from high-output cardiac failure secondary to chronic severe anemia, pulmonary AVMs with previous embolizations, and hysterectomy at the age of 26 for menorrhagia.
Her initial transfusion requirement was approximately one unit of packed red blood cells (pRBC) per month, but as of 2003 required > 40 units per year due to persistent epistaxis and increasing upper GI bleeding in the form of melena. Capsule endoscopies and upper endoscopies demonstrated innumerable AVMs in the esophagus, stomach, duodenum, and jejunum, with the bulk of the lesions located in the proximal small bowel where active bleeding was most frequently seen. Mesenteric angiography also demonstrated diffuse vascular abnormality/telangiectasia involving proximal small bowel but no focal AVM amenable to embolization. Biochemical investigations excluding other etiologies included normal renal function, thyroid stimulating hormone, haptoglobin, bilirubin, lactate dehydrogenase, direct agglutination testing, serum protein electrophoresis, serum free light chain, urine protein electrophoresis, and von Willebrand factor studies. Her hepatitis C viral load was low and abdominal ultrasound did not demonstrate cirrhosis.
Her transfusion requirement further escalated despite successful interventions including multiple septal dermatoplasties and facial/nasal vessel angiography with embolization. At least seven upper endoscopies with argon plasma coagulation, two-month tamoxifen therapy, and tranexamic acid which led to upper extremity deep vein thrombosis, were used for ongoing bleeding from the duodenum/proximal jejunum. She received neither estrogen therapy nor thalidomide due to risk of hormone-sensitive malignancy and limited access/financial constraint, respectively. Additionally, her advanced cardiopulmonary comorbidities, which were complications of HHT, precluded surgical resection of the proximal small bowel (i.e., Whipple procedure) containing the majority of the vascular lesions in order to reduce GI bleeding. Between 2008 and 2015, she received on average six units of pRBC weekly (i.e., 312 unit per year) as well as monthly intravenous iron infusion.
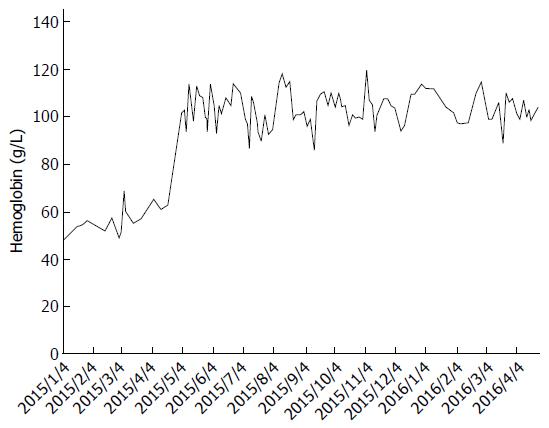
In April 2015, she was started on bevacizumab, an anti-VEGF antibody, at 5 mg/kg every two weeks as a last resort. The patient’s response to bevacizumab in terms of GI bleeding was immediate and dramatic: Her stools returned to normal color and consistency, and her transfusion requirement dropped to four units per month between May and September, and further down to only two units over the course of the following seven months with intravenous iron supplementation (Figure 1). She continued to experience minor epistaxis that was much less severe than previous, and ongoing microscopic hematuria without proteinuria or renal function impairment.
Over the past 12 mo, the administration of bevacizumab has resulted in a decrease of approximately 290 units of pRBC’s and improved the patient’s quality of life immensely. The patient has tolerated the infusions without any reported side-effects.
This case highlights the potential challenges in managing HHT with complicated, refractory GI bleeding. In our patient’s case, she acquired hepatitis C infection due to repeated transfusions, and developed high-output cardiac failure and pulmonary hypertension due to severe chronic anemia. Her functional capacity was poor due to anemia and pulmonary hypertension, but the need for frequent transfusions three times a week (> 1400 units of pRBC in the five years prior to treatment) also posed a tremendous negative impact on her quality of life in addition to causing a significant burden on the health care system. Fortunately, the patient’s response to bevacizumab exceeded our expectations. The greatest hurdle of obtaining the drug was in fact, obtaining approval from regulatory bodies for financial coverage of it. However, after one year, the overall cost to the health care system of avoiding most transfusions is staggering. Given the severity of her blood loss, which to our knowledge is one of the most severe cases reported, our initial goal had been to reduce her transfusion requirement by 40%-50%, but she essentially became transfusion-independent.
We opted to prescribe 5 mg/kg every other week, which is the typical dose for solid organ malignancies, since this is the regimen with the most clinical evidence to date for HHT[17]. The optimal dosage and duration of bevacizumab for GI bleeding in HHT remains to be determined, and requires balancing the clinical benefits against potential adverse events of systemic treatment such as hypertension, nephrotic syndrome, poor wound healing, bowel perforation, and thromboembolic events[18]. There have been reports successfully utilizing lower doses for GI bleeding, based on the drug’s pharmacokinetics[4,5,7,15], but some patients may require higher doses to maintain optimal response[10]. Although the specific optimal dosage is not entirely clear at this time, the duration of treatment is likely indefinite as relapses upon discontinuation of therapy have been noted in previous reports, with the interval ranging from one to three months after the last dose[4,7,8,15].
This case demonstrates a remarkable response in regards to GI bleeding to bevacizumab in the setting of HHT. Although not specifically examined yet in our patient, additional benefits such as partial reversal of liver failure and high-output cardiac failure have also been reported[19-21]. Despite its high cost, earlier administration should be considered if benefit of this extent is regularly demonstrated in this group of patients as prevention of end organ complications (i.e., cardiac/hepatic) theoretically might have been prevented with early administration. The issue of cost in many situations perhaps should not be paramount when aiming for ideal care in these patients with limited options.
Bevacizumab effectively controlled severe GI bleeding in this patient with complicated HHT.
A 58-year-old women with hereditary hemorrhagic telangiectasia (HHT) presented with severe, chronic gastrointestinal (GI) bleeding refractory to multiple treatment modalities.
Endoscopies demonstrated numerous arteriovenous malformations in the esophagus, stomach, duodenum, and jejunum. The majority of the lesions were located in the proximal small bowel where active bleeding was frequently seen.
Vascular lesions of GI tract: Angiodysplasia (sporadic, end-stage renal disease, aortic stenosis, von Willebrand disease, left ventricular assist device), systemic disease (CREST, HHT).
Biochemical investigations included normal renal function, thyroid stimulating hormone, haptoglobin, bilirubin, lactate dehydrogenase, direct agglutination testing, serum protein electrophoresis, serum free light chain, urine protein electrophoresis, and von Willebrand factor studies. Pre-bevacizumab mean hemoglobin was 57.2 ± 5.8 g/dL. Post-bevacizumab hemoglobin was 103.4 ± 8.3 g/dL.
Mesenteric angiography did not identify suitable arteriovenous malformation that was amenable to treatment via embolization of the gastroduodenal artery.
Bevacizumab 5 mg/kg infusion every other week.
There is growing evidence in the literature on bevacizumab’s efficacy in treating recurrent epistaxis due to HHT, but relatively few reports exist for the management of severe, chronic, refractory GI bleeding. This case highlights the potential utility of bevacizumab for such management.
HHT is an autosomal dominant condition characterized by vascular malformations that can occur anywhere in the body, leading to various presentations including epistaxis, GI bleeding, high-output cardiac failure, and hypoxemia.
Earlier administration of bevacizumab should be considered in patients with severe, refractory GI bleeding despite its relatively high cost given the potential benefit.
This is a pretty straightforward case report.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Field K S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Sadick H, Riedel F, Naim R, Goessler U, Hörmann K, Hafner M, Lux A. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica. 2005;90:818-828. [PubMed] |
| 2. | Wee JW, Jeon YW, Eun JY, Kim HJ, Bae SB, Lee KT. Hereditary hemorrhagic telangiectasia treated with low dose intravenous bevacizumab. Blood Res. 2014;49:192-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Thompson AB, Ross DA, Berard P, Figueroa-Bodine J, Livada N, Richer SL. Very low dose bevacizumab for the treatment of epistaxis in patients with hereditary hemorrhagic telangiectasia. Allergy Rhinol (Providence). 2014;5:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Suppressa P, Liso A, Sabbà C. Low dose intravenous bevacizumab for the treatment of anaemia in hereditary haemorrhagic telangiectasia. Br J Haematol. 2011;152:365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Sehl ME, M Gruber T, McWilliams JP, Marder VJ. Successful management of chronic gastrointestinal hemorrhage using bevacizumab in the setting of hereditary hemorrhagic telangiectasia. Am J Hematol. 2015;90:561-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Lazaraki G, Akriviadis E, Pilpilidis I, Parisi I, Tzilves D, Tarpangos A. Low dose of bevacizumab is safe and effective in preventing bleeding episodes in hereditary hemorrhagic telangiectasia. Am J Gastroenterol. 2011;106:2204-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kochanowski J, Sobieszczańska M, Tubek S, Żurek M, Pawełczak J. Successful therapy with bevacizumab in a case of hereditary hemorrhagic telangiectasia. Hum Vaccin Immunother. 2015;11:680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Fodstad P, Dheyauldeen S, Rinde M, Bachmann-Harildstad G. Anti-VEGF with 3-week intervals is effective on anemia in a patient with severe hereditary hemorrhagic telangiectasia. Ann Hematol. 2011;90:611-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Flieger D, Hainke S, Fischbach W. Dramatic improvement in hereditary hemorrhagic telangiectasia after treatment with the vascular endothelial growth factor (VEGF) antagonist bevacizumab. Ann Hematol. 2006;85:631-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Epperla N, Hocking W. Blessing for the bleeder: bevacizumab in hereditary hemorrhagic telangiectasia. Clin Med Res. 2015;13:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Brinkerhoff BT, Poetker DM, Choong NW. Long-term therapy with bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2011;364:688-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Brinkerhoff BT, Choong NW, Treisman JS, Poetker DM. Intravenous and topical intranasal bevacizumab (Avastin) in hereditary hemorrhagic telangiectasia. Am J Otolaryngol. 2012;33:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Bose P, Holter JL, Selby GB. Bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;360:2143-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Amanzada A, Töppler GJ, Cameron S, Schwörer H, Ramadori G. A case report of a patient with hereditary hemorrhagic telangiectasia treated successively with thalidomide and bevacizumab. Case Rep Oncol. 2010;3:463-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Lupu A, Stefanescu C, Treton X, Attar A, Corcos O, Bouhnik Y. Bevacizumab as rescue treatment for severe recurrent gastrointestinal bleeding in hereditary hemorrhagic telangiectasia. J Clin Gastroenterol. 2013;47:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Amann A, Steiner N, Gunsilius E. Bevacizumab: an option for refractory epistaxis in hereditary haemorrhagic telangiectasia. Wien Klin Wochenschr. 2015;127:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Arizmendez NP, Rudmik L, Poetker DM. Intravenous bevacizumab for complications of hereditary hemorrhagic telangiectasia: a review of the literature. Int Forum Allergy Rhinol. 2015;5:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Vlachou PA, Colak E, Koculym A, Kirpalani A, Kim TK, Hirschfield GM, Faughnan ME. Improvement of ischemic cholan–giopathy in three patients with hereditary hemorrhagic telangiectasia following treatment with bevacizumab. J Hepatol. 2013;59:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E, Roux A, Carette MF, Gilbert-Dussardier B, Hatron PY. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Mitchell A, Adams LA, MacQuillan G, Tibballs J, vanden Driesen R, Delriviere L. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transpl. 2008;14:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |