Published online Dec 27, 2016. doi: 10.4240/wjgs.v8.i12.770
Peer-review started: June 28, 2016
First decision: August 5, 2016
Revised: September 27, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 27, 2016
Processing time: 177 Days and 19.3 Hours
To investigate feasibility and outcome of abdominal-sacral resection for treatment of locally recurrent rectal adenocarcinoma.
A population of patients who underwent an abdominal-sacral resection for posterior recurrent adenocarcinoma of the rectum at the National Cancer Institute of Milano, between 2005 and 2013, is considered. Retrospectively collected data includes patient characteristics, treatment and pathology details regarding the primary and the recurrent rectal tumor surgical resection. A clinical and instrumental follow-up was performed. Surgical and oncological outcome were investigated. Furthermore an analytical review of literature was conducted in order to compare our case series with other reported experiences.
At the time of abdomino-sacral resection, the mean age of patients was 55 (range, 38-64). The median operating time was 380 min (range, 270-480). Sacral resection was performed at S2/S3 level in 3 patients, S3/S4 in 3 patients and S4/S5 in 4 patients. The median operating time was 380 ± 58 min. Mean intraoperative blood loss was 1750 mL (range, 200-680). The median hospital stay was 22 d. Overall morbidity was 80%, mainly type II complication according to the Clavien-Dindo classification. Microscopically negative margins (R0) is obtained in all patients. Overall 5-year survival after first surgical procedure is 60%, with a median survival from the first surgery of 88 ± 56 mo. The most common site of re-recurrence was intrapelvic.
Sacral resection represents a feasible approach to posterior rectal cancer recurrence without evidence of distant spreading. An accurate staging is essential for planning the best therapy.
Core tip: During the last years, great efforts have been invested by many authors to contribute in treatment of rectal cancer recurrence without evidence of distant spreading. The most difficult surgical problem is to perform an affective radical R0 salvage resection. However, with the introduction of sacral resection, consistent improvements have been achieved in recent years, particularly when local tumor relapse occurs in the posterior part of the pelvis, from the presacral to the retrovescical spaces. However, abdominosacral resection is a complex surgical procedure affected by several postoperative complications. For this reason, these patients should be treated into dedicated and specialized institutions.
- Citation: Belli F, Gronchi A, Corbellini C, Milione M, Leo E. Abdominosacral resection for locally recurring rectal cancer. World J Gastrointest Surg 2016; 8(12): 770-778
- URL: https://www.wjgnet.com/1948-9366/full/v8/i12/770.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i12.770
Local relapse of rectal cancer is still one of the most complex and challenging issue concerning surgical oncology of the last years. Indeed, nowadays, rectal cancer relapses during the first two years in the 7%-30% of patients after receiving surgical resection[1-3]. In about half of these cases of relapse, the cancer remains confined in the pelvis without extraregional diffusion and most of the deceases associated to the disease are only due to local progression of it in the following periods.
The infiltration of the pelvic walls had represented up to recent years the main limitation to achieve a radical resection in the majority of relapsed rectal cancer cases. Nevertheless, the recent advancements in surgical techniques especially regarding posterior and anterior relapse resection, have widen up the spectrum of possibilities for effective curative treatment[4-6].
With the aim of contribute in the field, we present the review of the literature and report the implications from the experience obtained at our hospital, the National Cancer Institute of Milan, on abdomino-sacral resection (ASR) for pelvic posterior recurrences of rectal cancers expanding toward the sacral plane.
Between 2005 and 2013 in our Unit 1324 patients affected with rectal cancer were treated with different surgical procedures. One hundred and sixty-two (12.2%) recurred in the pelvis in a period ranging from 10 to 38 mo after surgery. One hundred and fifty-four of these were considered candidates to a second surgical salvage approach. Different surgical procedures were applied accordingly with the extension, the site and the characteristic of the relapsing lesion.
In the same period, a population of ten patients underwent an ASR for recurrent adenocarcinoma of the rectum at the National Cancer Institute of Milano. These patients are included in the present study.
All the patients underwent, in the first place, a radical resection for the rectal cancer followed by at our Institution in combination with a total mesorectal excision (TME) and a local, nerve sparing, node dissection extended to the origin of low mesenteric vessels. Local recurrence is defined as the relapse of the tumour at the primary site confirmed by radiologically and/or histologically. In all cases the recurrence was mainly posterior and invading the presacral space or directly the sacral plane.
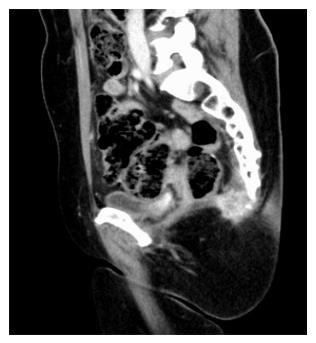
Indications for ASR exist when there is evidence of involvement of the sacrum detected by preoperative exams (Figure 1) or when there is a highly probability of it according with the pelvic local extension of local relapse.
Patients were staged preoperatively by a thoraco-abdominal computed tomography (CT)-scan, a pelvic magnetic resonance imaging (MRI), a positron emission tomography and, when possible, a colonoscopy. The study excluded patients whose recurrent rectal cancer was developed after a simple local excision or patients receiving a simple and limited coccyx resection.
Patients who had undergone resection of liver metastasis at initial surgery or before the diagnosis of local relapse were also considered suitable for ASR, given an adequately long distant metastasis-free survival period. All data are retrospectively collected and registered prospectively into an electronic database. Collected data includes patient characteristics, treatment and pathology of the primary rectal cancer, neoadjuvant therapy and operative details for recurrent tumor, pathology of recurrent tumor, length of hospital stay, peri-operative complications, blood transfusion needed and oncological outcome.
The macroscopic and microscopic assessments of the pathology specimens were done by a single pathologist at our Hospital. Pathological examination included histological type, number of lymph nodes harvested, number of metastatic lymph nodes, analysis of specimen resection margins and evaluation of sacral involvement. An R0 resection is defined when no tumour cells are shown in the surgical resection margin. Pathologic stage information was assessed using the 7th edition of the American Joint Committee on Cancer TNM system.
Surgical complications and overall morbidity complications are defined as adverse events that occurred within a 30 d period after surgery. Surgical complications are staged according to the Claviene Dindo Classification.
After ASR, clinical and instrumental evaluations were performed every 6 mo for the first three years and one a year for the following years. Computed axial CT-scan and MRI surveillance, as well as carcinoembryonic antigen levels, were the exams performed to assess patient outcome.
The surgical procedure is divided in three following steps: Abdominal, perineal and sacral.
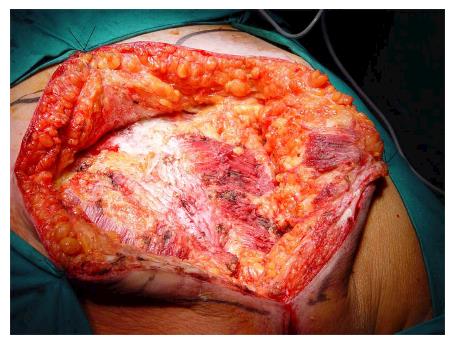
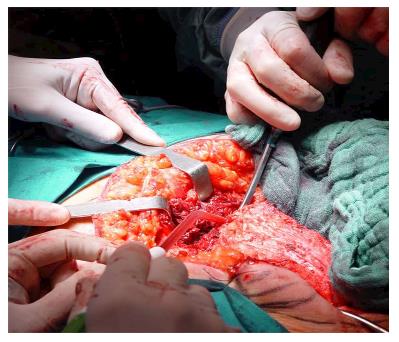
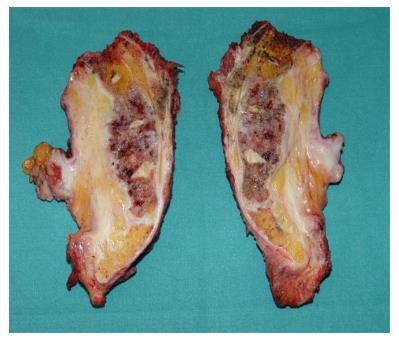
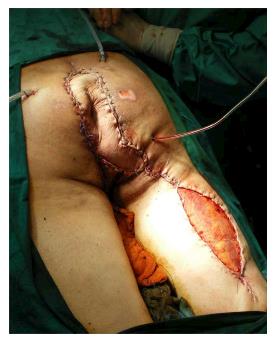
Under general anesthesia the patient was placed in Lloyd-Davies position (lithotomic position with flexed and abducted thighs). After placement of ureteral stents (4 cases) in order to identify the ureters, a midline laparotomy was performed. The dissection of common and external iliac vessels is started from the promontorium. The anterior area from the aortic bifurcation to the sacral promontory is exposed to have access to the anterior surface of the sacrum. The dissection is made down to the distal sacrum paying the upmost attention to avoid bleeding from the prescral space that in some case could be really important. The area from the common iliac artery to the bifurcation between the internal and external iliac arteries is exposed. The dissection is then made toward the presacral space along the parietal pelvic fascia, outside the original plane of dissection. During this phase the endopelvic fascia and pubo-prostatic ligaments can be identified bilaterally and divided using electric cautery to expose the levator ani muscle. Some Authors propose preventive ligation of internal iliac vessels along the sacral plane, in order to control the risk of bleeding during surgical dissection. However, this procedure was not routinely performed in our series because we believe that this approach is needed only in upper sacral resection due to a higher risk of local bleeding. The perineal phase corresponds to the typical procedure adopted in an abdomino-perineal resection performed for a primary rectal or anal carcinoma but avoiding to remove the surgical specimen through the perineal wound because the rectum and the other tissues will be removed “en block” with the sacrum during the following surgical steps. After formation of a terminal colostomy and closure of abdominal wound, the patient is placed in a prone position, with flexed and abducted thighs. Then, a posterior midline incision including the perineal lesion is made. The gluteus maximus muscles are dissected and detached from the sacrum in order to obtain a full exposure (Figure 2). The next step of this phase involves detaching the gluteal muscles, the sacrotuberous and sacrospinous ligaments and the piriform muscle from the sacrum to, subsequently, access the pelvic cavity. The surgeon inserts an index finger into the pelvic cavity from the lower edge of the sacroiliac joint and checks the dissected level of the anterior surface of the sacrum to determine the level of sacral amputation. The posterior wall of the sacrum is then osteotomized using a proper chisel and hammer at a stretch (Figure 3) scalpel and en-bloc resection of the tumor with the sacrum and the surrounding organs is accomplished (Figure 4). The canal is sealed with bone wax and fibrin sealant. A prolene mesh is placed anterior to the sacrum in order to close the perineal defect. A primary wound closure is usually performed. In some cases (two patients of the present series), perineal reconstruction is achieved with a pedicled musculocutaneous flap (Figure 5).
All ten patients included in this study (4 males and 6 females) underwent an anterior rectal resection as first operation at our hospital. Patients characteristics are reported in Table 1.
| Case | Age | Sex | First surgery | Pathological stage | DFS (mo) | OS1 (mo) | OS2 (mo) | Status |
| 1 | 63 | F | ARR | pT3 pN0 M0 | 103 | 216 | 111 | DOD (local and lung recurrences) |
| 2 | 60 | F | ARR | pT2 pN0 M0 | 89 | 135 | 41 | NED |
| 3 | 62 | F | ARR | pT2 pN0 M0 | 114 | 154 | 34 | NED |
| 4 | 53 | F | ARR | pT2 pN1 M0 | 76 | 93 | 22 | DOD (local recurrence) |
| 5 | 46 | M | ARR | pT3 pN1 M0 (R1) | 12 | 54 | 41 | DOD (local and lung recurrences) |
| 6 | 64 | F | ARR | pT2 pN0 M0 | 22 | 83 | 57 | NED |
| 7 | 57 | F | ARR | pT3 pN2 M0 | 16 | 38 | 13 | DOD (liver recurrence) |
| 8 | 47 | M | ARR and liver metastasectomy | pT3 pN1 M1 (liver) | 29 | 47 | 14 | DOD (local recurrence) |
| 9 | 38 | M | ARR | pT2 pN0 M0 | 49 | 110 | 57 | NED |
| 10 | 57 | M | ARR | pT2 pN0 M0 | 17 | 56 | 29 | NED |
All the tumors were adenocarcinomas. Median distance from anal verge was 6 cm (range, 3-11). No patient received a pre-surgical neo-adjuvant therapy. In the pT3 cases this was mainly due to the bad clinical status of the patients at the moment of diagnosis (occluding or bleeding lesions). All patients with histological nodal involvement or diffusion of disease into the perirectal fat received a post-operative CT-RT treatment in accordance to standardized schedules. The results of the pathological staging according to TNM are as follows: Stage I (5 cases), stage IIa (1 case), stage IIIa (1 case), stage IIIb (2 case) and stage IVa (1 case). In our case series, all stage I reported were high grade tumors with associated vascular and perineural invasion. Radicality was achieved during first surgery in all cases excepting one (R1, case 5).
At the time of ASR, the mean age of patients was 55 ± 9 years old, ranging from 38 to 64 years old. One out of ten patients was asymptomatic at presentation while 9 patients had increasing lower sacrococcygeal region pain. All patients were free of distant metastases at ASR and were considered eligible for a radical resection. In 6 patients surgery was performed 4 to 6 wk after the completion of a new chemo-radiation therapy. All patients underwent ASR according to the technique reported in the previous section. The median operating time was 380 ± 58 min (range, 270-480 min). In one case, a posterior colpectomy was needed due to a direct infiltration of posterior vaginal wall (Table 2). Sacral resection was performed at S2/S3 level in 3 patients, S3/S4 in 3 patients and S4/S5 in 4 patients.
| Pre-ASRCT-RT | Surgical procedure | Level of sacrectomy | Procedure lenght (min) | In-hospital stay (d) | Sacral involvement | Early complications(within 30 d) | Late complications(after 30 d) | |
| 1 | Yes | ASR | S2-S3 | 380 | 11 | Yes | Neurologic bladder dysfunction | Perineal wound infection and leakage |
| 2 | Yes | ASR | S4-S5 | 400 | 10 | Yes | Pelvic abscess | Uterus/bladder prolapsus |
| 3 | Yes | ASR | S2-S3 | 420 | 24 | Yes | Perineal wound leakage and Neurologic bladder | No |
| 4 | No | ASR | S4-S5 | 370 | 10 | Yes | Perineal wound leakage | No |
| 5 | No | ASR | S3-S4 | 430 | 93 | No | Uretral fistula, perineal flap necrosis and wound leakage | No |
| 6 | Yes | ASR | S3-S4 | 360 | 25 | No | Perineal wound leakage | No |
| 7 | CT only | ASR and posterior colpectomy | S3-S4 | 360 | 8 | No | Perineal wound leakage | Pelvic abscess and ileal fistula |
| 8 | No | ASR | S4-S5 | 480 | 11 | Yes | No | No |
| 9 | Yes | ASR | S2-S3 | 270 | 9 | No | No | No |
| 10 | Yes | ASR | S4-S5 | 330 | 15 | No | Perineal wound infection | No |
In our case series, overall morbidity was 80%, i.e., 5 presented postoperative type II complications, 2 had type IIIb and one 1 a type IIIa complication according to the Clavien-Dindo classification, whereas the most frequent complication (7 patients) was sacral wound skin infection and dehiscence. List of main complications are reported in Table 2. No differences in complication rates between higher and lower sacral resection are detected. Mean intraoperative blood loss was 1750 mL (range, 200-6800 mL). Blood transfusion was administered to 5 patients (median 1 unit; range, 0-8 units) during the surgical procedure and to 5 patients (median 2 units, range, 0-8 units) during the postoperative period. No in-hospital mortality was observed. The median hospital stay was 22 d (range, 8-93 d). Adjuvant chemotherapy was performed in 2 patients under suggestion of medical oncologists due to local extension of disease. Microscopically negative margins (R0) is obtained in all patients. Previous diagnosis was confirmed by histopathological examination. Sacral bone invasion was detected in 50% of the cases. Postoperative lymph node harvested are 4 ± 3 (range, 0-10). No node localizations are identified.
Five of 10 patient (50%) died at a median time from ASR of 38 ± 29 mo.
Overall 5-year survival after first surgical procedure is 60%, with a median survival from the first surgery of 88 ± 56 mo. Median disease free interval from first surgery to local recurrence is 39 ± 39 mo (range 12-114 mo). The most common site of re-recurrence was intrapelvic in 4 patients and two of these presented also lung metastases. One patient had liver metastasis alone.
The adoption of TME, described by MacFarlane et al[7] associated with preoperative radiation has improved surgical management of primary rectal cancer leading to a significant decrease of locoregional recurrence from 33% to less than 10%[8,9].
Despite these recent advancements, the occurrence of local relapse of rectal cancer is still quite frequent and produces a particular cancer situation characterized by the persistence of the disease in the pelvis without extraregional diffusion to distant sites. The specific behavior of this recurring tumor calls for the need of an advanced surgical or combined therapy in order to obtain a R0 resection in the pelvis.
Regretfully, these locoregional recurrences are often spread through the whole pelvis and a salvage surgery cannot be attempted. As a result many of them die in very bad conditions only for progression of the local relapse.
Several risk factors for the recurrence of rectal tumors have been studied. Some of them are related to tumor features, including tumor localization and pathological stage[10]. However, the main risk factors are linked to which and how surgical technique is performed, e.g., incomplete resection of mesorectal fatty tissue and R1/R2 resection[11]. This explains why up to 90% of these relapses occur in an extra-bowel site and justify the difficulties of diagnosis and complexity of surgical resection for the adhesion-infiltration of these recurrences to the pelvic structures.
An accurate locoregional staging of a rectal relapsing tumor is essential for planning the best therapy[12-14]. A careful radiological examination provides information about the local extension of the disease, which is critical for the treatment decision-making process. A attentive evaluation of both tumor extent and anatomic planes is needed to determine a correct line during resection that is usually altered by the previous surgery and radiotherapy. Pelvic MRI and CT-scanning of the thorax and abdomen are the most used imaging modalities technique in pre-operative staging to evaluate whether or not curative surgery is feasible, although some authors underline a low sensitivity in accurate assessment of side wall involvement[15,16].
The definition of the site distribution of the relapse is crucial because, from a practical point of view, the factor that seems to play the upmost relevant role in evaluating the surgical resectability of these peculiar lesions is the anatomical sites of recurrences in the pelvis, irrespective in many cases, of the dimension and time of occurrence.
The relevance of the sites of recurrences is confirmed by the effort that has been dedicated to this issue in all the schemes of classifications proposed in the past recent years[17-21]. Guillem et al[19] in 1998 classified relapses into four groups: Axial, anterior, posterior and lateral. Furthemore, Guillem’s classification was adopted by Moore et al[21] to show that the likelihood of achieving a R0 resection is strongly correlated to the type of recurrence, reporting an higher R0 resection rate in axial and anterior lesions than in lateral and posterior ones. Others studies confirm that central or anterior localization of a relapse produced less complex difficulties due to the possibility of removing pelvic organs such as uterus, vagina or bladder by means of well defined procedures[22-24]. With respect to lateral and posterior relapses, a crucial role is played by the presence/absence of infiltration of structures such as iliac vessels, ureters, bony pelvis and great sciatic notch. Extensive infiltration of the pelvic sidewall is also a poor prognostic factor for the oncological outcome[25,26].
There are, anyway, bone sections that can be removed through complex surgical procedures with relatively limited functional consequences. This concerns, specifically, the middle and distal portion of the sacrum. Facing this technical problem there are many points that should be considered.
The first one is defining the level of bone transection to be done that must be at least 1-2 cm above the upper edge of the visible tumor, when possible. As a matter of fact, the level of resection in almost all published series remains as the key factor, influencing the neurological and intraoperative complications rate[27-29]. In all generality, there are not absolute limitation even to the resection of the whole sacrum from S1 to the coccyx, but this massive resection has been considered an alternative in rare situations, mainly due to the complexity of the procedure and to the functional consequences that may affect the patient against the expected limited benefit for the extension of the disease[30,31]. Regularly, the section of neural roots at S3 level has no main sequelae and it is well accepted. Moving to S2 the cutting of the second root could produce important modification of bladder function up to a complete loss of bladder motility. Upper sections produce remarkable lower limb motor disability and plantar flexion weakness and the need of a walking aid. Commonly, the level considered as acceptable limit for this type of surgery is the space between S2 and S3; this allows classifying as “high sacrectomy” all resections extended from the space between S2 and S3 and, as “low sacretomy”, the procedures performed below this level[18,26,32]. Other authors suggest a different classification indicating as “high sacrectomy” resection from the intervertebral disk between S1 and S2, “middle sacrectomy” resections between S2 and S3, and “low sacrectomy” all the others below[33]. High sacrectomy are indeed followed by significant complications and morbidity. Bhangu et al[23] reports a 60% incidence of major complications for S1-S2 resection in comparison to a 27%-29% rate for S3 and S4-S5 sacrectomy. These data have been strongly confirmed in the recent years by many others studies[1,6,34-38].
A other relevant technical aspect concerns the intraoperative complications related to this difficult surgical procedure with special regards to the occurrence of sacral bleeding. Furthermore, in this case, the level of transaction is directly correlated to the incidence and severity of venous blood loss that, in some cases, could be not easily controlled, even become life threatening. Intraoperative bleeding during this surgery can be sudden and severe, and more often in patients who underwent preoperative radiotherapy, as frequently observed in these cases[39-42].
The postoperative period could also be compromized by different and complex problems. As indicated in several studies, perioperative complication rate is high, especially in upper sacral resections. Morbidity and mortality rate at three months after radical ASR for recurrence are reported to be 30% and 8%, respectively[1,6,34-36]. The most common complications occurring after sacropelvic resection are wound infections and dehiscences, pelvic abscesses and clinical complication, like as pneumonia, urinary tract infections and sepsis[6]. Between the 15%-58% local complication rate (wound dehiscence, pelvic sepsis, flap necrosis, etc.) are justified by a modified and affected wound healing processes of the perineal and sacral zone. This event is often due to a heavy and prolonged radiotherapy treatment applied to the perineum and to the sacral area following rectal resection[6,23,29,36]. All these occurrences explain as well the need to perform, in many of these cases, different and elaborated plastic reconstructions (mio-cutaneos or fascial-cutaneous flaps, rotation flaps or others) or more specific procedures when the site of the disease request further and more extended demolitions, e.g., the vaginal or bladder areas[37].
Up to few years ago, the diagnosis of a local recurrence was strongly correlated to a poor prognosis with a mean 5 years overall survival not greater than 10%[26]. However, despite all these technical difficulties, the application of correct, enlarged and radical procedures have achieved positive and consistent clinical results in terms of local control of disease and improvements in the final outcome of these patients.
Recently, many authors have contributed to this topic, most of them confirming the safety of these surgical approaches when performed by dedicate and experienced groups of physicians. Several studies have demonstrated a 5-year global survival rates in local relapses surgery ranging between 25% and 60%[1,6,30-36], rate confirmed by our study in which out of 10 operated patients, all submitted a middle-distal resection of sacral body, 50% are currently alive with a 38 mo mean follow-up from ASR.
In order to increase radicality rate and have a better local control, neoadjuvant treatment may be useful, excepting patients who had previously received high dose radiotherapy for primary cancer or other diseases[43-45]. The role of intraoperative radiotherapy in the treatment of patients with pelvic relapses is still under discussion, notwithstanding several studies have shown the benefits in survival, especially in recurrent unresectable rectal tumors due to bone involvement. Some authors have reported an increase survival rate of 15% when this modality of radiotherapy is performed[43].
Despite all these particular aspects, the key issue to be addressed when managing this kind of disease is the appropriate selection of treatment by the patient in order to achieve symptom control and even a curative treatment with acceptable morbidity. When presenting a surgical alternative to the patient, the specialist should take into account several prognostic factors including: The age and comorbities of the patient, disease free interval and features of the recurrence of tumor presented earlier.
The most recent studies, as well as the one presented here, are highlighting that partial sacrectomy can be considered a safe and feasible approach for recurrent rectal cancer but also that such a complex surgical resection must only be considered if a radical resection is technically possible[37,46-53] on the basis of a multidisciplinary team evaluation only into dedicated and specialized institutions.
The results obtained in our series are consistent with what reported in other studies and suggest that, currently, a 5 years survival up to 60% is achieved with an acceptable morbidity and minor functional failure, when partial sacrectomy, below S2 level, is performed.
Local relapses of rectal cancer remain one of the most complex and challenging aspects of surgical oncology of the last years.
Recently, a consistent improvement has been achieved with the introduction of sacral resection for the patients with posterior pelvic extension of the recurrent tumor. Although abdominosacral resection could improve outcome in patients affected by rectal cancer recurrence, it is been reported to be a complex surgical procedure affected by several postoperative complications.
Reporting their experience, the authors would like to give a better comprehension of a challenging disease with some suggestions about its surgical management.
The results of this study support the previously published evidences, underling the indication to performed a carefully selection of patient to treat. Further researches are needed to improve surgical technique and patient selection.
This is an interesting article on a limited series of a surgical procedure which is not often performed for treatment of local recurrent rectal cancer. Authors report data from their own experience and also make a review of the literature on this subject.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Carboni F, Hotta T, Kapoor S, Lirici MM S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Hogan NM, Joyce MR. Surgical management of locally recurrent rectal cancer. Int J Surg Oncol. 2012;2012:464380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Jensen LH, Altaf R, Harling H, Jensen M, Laurberg S, Lindegaard JC, Muhic A, Vestermark L, Jakobsen A, Bülow S. Clinical outcome in 520 consecutive Danish rectal cancer patients treated with short course preoperative radiotherapy. Eur J Surg Oncol. 2010;36:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3119] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 4. | Salo JC, Paty PB, Guillem J, Minsky BD, Harrison LB, Cohen AM. Surgical salvage of recurrent rectal carcinoma after curative resection: a 10-year experience. Ann Surg Oncol. 1999;6:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Cohen AM, Minsky BD. Aggressive surgical management of locally advanced primary and recurrent rectal cancer. Current status and future directions. Dis Colon Rectum. 1990;33:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Moriya Y, Akasu T, Fujita S, Yamamoto S. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis Colon Rectum. 2004;47:2047-2053; discussion 2047-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1221] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 8. | Read TE, Mutch MG, Chang BW, McNevin MS, Fleshman JW, Birnbaum EH, Fry RD, Caushaj PF, Kodner IJ. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Delpero JR, Lasser P. [Curative treatment of local and regional rectal cancer recurrences]. Ann Chir. 2000;125:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Syk E, Torkzad MR, Blomqvist L, Nilsson PJ, Glimelius B. Local recurrence in rectal cancer: anatomic localization and effect on radiation target. Int J Radiat Oncol Biol Phys. 2008;72:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Kapiteijn E, van de Velde CJ. The role of total mesorectal excision in the management of rectal cancer. Surg Clin North Am. 2002;82:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Titu LV, Nicholson AA, Hartley JE, Breen DJ, Monson JR. Routine follow-up by magnetic resonance imaging does not improve detection of resectable local recurrences from colorectal cancer. Ann Surg. 2006;243:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 14. | Tan PL, Chan CL, Moore NR. Radiological appearances in the pelvis following rectal cancer surgery. Clin Radiol. 2005;60:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kim DJ, Kim JH, Lim JS, Yu JS, Chung JJ, Kim MJ, Kim KW. Restaging of Rectal Cancer with MR Imaging after Concurrent Chemotherapy and Radiation Therapy. Radiographics. 2010;30:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Valentini V, Morganti AG, De Franco A, Coco C, Ratto C, Battista Doglietto G, Trodella L, Ziccarelli L, Picciocchi A, Cellini N. Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma: prognostic factors and long term outcome. Cancer. 1999;86:2612-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Wanebo HJ, Antoniuk P, Koness RJ, Levy A, Vezeridis M, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Guillem JG, Ruo L. Strategies in operative therapy for locally recurrent rectal cancer. Semin Colon Rectal Surg. 1998;9:259-268. |
| 20. | Suzuki K, Dozois RR, Devine RM, Nelson H, Weaver AL, Gunderson LL, Ilstrup DM. Curative reoperations for locally recurrent rectal cancer. Dis Colon Rectum. 1996;39:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Moore HG, Shoup M, Riedel E, Minsky BD, Alektiar KM, Ercolani M, Paty PB, Wong WD, Guillem JG. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Heriot AG, Tekkis PP, Darzi A, Mackay J. Surgery for local recurrence of rectal cancer. Colorectal Dis. 2006;8:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Bhangu A, Brown G, Akmal M, Tekkis P. Outcome of abdominosacral resection for locally advanced primary and recurrent rectal cancer. Br J Surg. 2012;99:1453-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Akasu T, Yamaguchi T, Fujimoto Y, Ishiguro S, Yamamoto S, Fujita S, Moriya Y. Abdominal sacral resection for posterior pelvic recurrence of rectal carcinoma: analyses of prognostic factors and recurrence patterns. Ann Surg Oncol. 2007;14:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Brunschwig A, Barber HR. Pelvic exenteration combined with resection of segments of bony pelvis. Surgery. 1969;65:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Wanebo HJ, Marcove RC. Abdominal sacral resection of locally recurrent rectal cancer. Ann Surg. 1981;194:458-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Madoff RD. Extended resections for advanced rectal cancer. Br J Surg. 2006;93:1311-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Weber KL, Nelson H, Gunderson LL, Sim FH. Sacropelvic resection for recurrent anorectal cancer. A multidisciplinary approach. Clin Orthop Relat Res. 2000;42:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Fawaz K, Smith MJ, Moises C, Smith AJ, Yee AJ. Single-stage anterior high sacrectomy for locally recurrent rectal cancer. Spine (Phila Pa 1976). 2014;39:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Ramamurthy R, Bose JC, Muthusamy V, Natarajan M, Kunjithapatham D. Staged sacrectomy--an adaptive approach. J Neurosurg Spine. 2009;11:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Milne T, Solomon MJ, Lee P, Young JM, Stalley P, Harrison JD. Assessing the impact of a sacral resection on morbidity and survival after extended radical surgery for locally recurrent rectal cancer. Ann Surg. 2013;258:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Dozois EJ, Privitera A, Holubar SD, Aldrete JF, Sim FH, Rose PS, Walsh MF, Bower TC, Leibovich BC, Nelson H. High sacrectomy for locally recurrent rectal cancer: Can long-term survival be achieved? J Surg Oncol. 2011;103:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Uehara K, Ito Z, Yoshino Y, Arimoto A, Kato T, Nakamura H, Imagama S, Nishida Y, Nagino M. Aggressive surgical treatment with bony pelvic resection for locally recurrent rectal cancer. Eur J Surg Oncol. 2015;41:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Mannaerts GH, Rutten HJ, Martijn H, Groen GJ, Hanssens PE, Wiggers T. Abdominosacral resection for primary irresectable and locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Heriot AG, Byrne CM, Lee P, Dobbs B, Tilney H, Solomon MJ, Mackay J, Frizelle F. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Melton GB, Paty PB, Boland PJ, Healey JH, Savatta SG, Casas-Ganem JE, Guillem JG, Weiser MR, Cohen AM, Minsky BD. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Dis Colon Rectum. 2006;49:1099-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Sagar PM, Gonsalves S, Heath RM, Phillips N, Chalmers AG. Composite abdominosacral resection for recurrent rectal cancer. Br J Surg. 2009;96:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Ferenschild FT, Vermaas M, Verhoef C, Dwarkasing RS, Eggermont AM, de Wilt JH. Abdominosacral resection for locally advanced and recurrent rectal cancer. Br J Surg. 2009;96:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Wang QY, Shi WJ, Zhao YR, Zhou WQ, He ZR. New concepts in severe presacral hemorrhage during proctectomy. Arch Surg. 1985;120:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Hill AD, Menzies-Gow N, Darzi A. Methods of controlling presacral bleeding. J Am Coll Surg. 1994;178:183-184. [PubMed] |
| 41. | Lou Z, Zhang W, Meng RG, Fu CG. Massive presacral bleeding during rectal surgery: from anatomy to clinical practice. World J Gastroenterol. 2013;19:4039-4044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Baqué P, Karimdjee B, Iannelli A, Benizri E, Rahili A, Benchimol D, Bernard JL, Sejor E, Bailleux S, de Peretti F. Anatomy of the presacral venous plexus: implications for rectal surgery. Surg Radiol Anat. 2004;26:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Mirnezami R, Chang GJ, Das P, Chandrakumaran K, Tekkis P, Darzi A, Mirnezami AH. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol. 2013;22:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Kusters M, Holman FA, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, van den Berg HA, van den Brule AJ, van de Velde CJ, Rutten HJ. Patterns of local recurrence in locally advanced rectal cancer after intra-operative radiotherapy containing multimodality treatment. Radiother Oncol. 2009;92:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Hansen MH, Balteskard L, Dørum LM, Eriksen MT, Vonen B. Locally recurrent rectal cancer in Norway. Br J Surg. 2009;96:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Rahbari NN, Ulrich AB, Bruckner T, Münter M, Nickles A, Contin P, Löffler T, Reissfelder C, Koch M, Büchler MW. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: is there still a chance for cure? Ann Surg. 2011;253:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Mannaerts GH, Martijn H, Crommelin MA, Stultiëns GN, Dries W, van Driel OJ, Rutten HJ. Intraoperative electron beam radiation therapy for locally recurrent rectal carcinoma. Int J Radiat Oncol Biol Phys. 1999;45:297-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Georgiou PA, Bhangu A, Brown G, Rasheed S, Nicholls RJ, Tekkis PP. Learning curve for the management of recurrent and locally advanced primary rectal cancer: a single team’s experience. Colorectal Dis. 2015;17:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Wells BJ, Stotland P, Ko MA, Al-Sukhni W, Wunder J, Ferguson P, Lipa J, Last L, Smith AJ, Swallow CJ. Results of an aggressive approach to resection of locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Ghouti L, Pereira P, Filleron T, Humeau M, Guimbaud R, Selves J, Carrere N. Pelvic exenterations for specific extraluminal recurrences in the era of total mesorectal excision: is there still a chance for cure?: a single-center review of patients with extraluminal pelvic recurrence for rectal cancer from March 2004 to November 2010. Am J Surg. 2015;209:352-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Troja A, El-Sourani N, Abdou A, Antolovic D, Raab HR. Surgical options for locally recurrent rectal cancer--review and update. Int J Colorectal Dis. 2015;30:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |