Published online Oct 27, 2016. doi: 10.4240/wjgs.v8.i10.706
Peer-review started: May 19, 2016
First decision: June 6, 2016
Revised: July 6, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: October 27, 2016
Processing time: 159 Days and 18.2 Hours
To investigate the feasibility of preoperative docetaxel, cisplatin and capecitabine (DCC) in patients with resectable gastric cancer.
Patients with resectable gastric cancer fulfilling the inclusion criteria, were treated with 4 cycles of docetaxel (60 mg/m2), cisplatin (60 mg/m2) and capecitabine (1.875 mg/m2 orally on day 1-14, two daily doses) repeated every three weeks, followed by surgery. Primary end point was the feasibility and toxicity/safety profile of DCC, secondary endpoints were pathological complete resection rate and pathological complete response (pCR) rate.
All of the patients (51) were assessable for the feasibility and safety of the regimen. The entire preoperative regimen was completed by 68.6% of the patients. Grade III/IV febrile neutropenia occurred in 10% of all courses. Three patients died due to treatment related toxicity (5.9%), one of them (also) because of refusing further treatment for toxicity. Of the 45 patients who were evaluable for secondary endpoints, four developed metastatic disease and 76.5% received a curative resection. In 3 patients a pCR was seen (5.9%), two patients underwent a R1 resection (3.9%).
Four courses of DCC as a preoperative regimen for patients with primarily resectable gastric cancer is highly demanding. The high occurrence of febrile neutropenia is of concern. To decrease the occurrence of febrile neutropenia the prophylactic use of granulocyte colony-stimulating factor (G-CSF) should be explored. A curative resection rate of 76.5% is acceptable. The use of DCC without G-CSF support as preoperative regimen in resectable gastric cancer is debatable.
Core tip: The use of the combination of docetaxel, cisplatin and capecitabine in resectable gastric cancer has resulted in a high curative resection rate of 77%, although it also resulted in a high rate of febrile neutropenia, and in treatment related mortality.
- Citation: Dassen AE, Bernards N, Lemmens VE, Wouw YAV, Bosscha K, Creemers GJ, Pruijt HJ. Phase II study of docetaxel, cisplatin and capecitabine as preoperative chemotherapy in resectable gastric cancer. World J Gastrointest Surg 2016; 8(10): 706-712
- URL: https://www.wjgnet.com/1948-9366/full/v8/i10/706.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i10.706
Although declining, gastric cancer is still ranking in the top 5 of incidence and mortality rates of malignancies in Europe[1]. Loco-regional and metastatic recurrence rates are high and prognosis remains poor, with a 5-year survival rate of 20%-31% for stage I-III disease[2]. Surgery is still the cornerstone of treatment for gastric cancer, although survival can be improved by adding perioperative treatment. In 2006, the results of the MAGIC trial were published showing that perioperative chemotherapy with epirubicin-cisplatin-5-fluorouracil (FU) (ECF) improved survival compared to surgery alone (5-year survival 36% vs 23%, respectively). Although most patients assigned to the perioperative chemotherapy tolerated the preoperative chemotherapy well, only 55% of them started the postoperative chemotherapy due to postoperative complications with only 42% of the patients completing the entire regimen[3]. These results demonstrate the problems encountered with the perioperative approach, i.e., many patients do not complete the full number of post-operative chemotherapy cycles.
In an attempt to increase efficacy and tolerability of chemotherapy regimen in gastric cancer other cytotoxic agents have been explored. The combination of docetaxel, cisplatin and fluorouracil has shown to be effective in advanced gastric cancer with reported overall response rates of 37%-43% and an acceptable safety profile[4-6]. Capecitabine, an orally substitute of 5-FU, offers a clear advantage in terms of convenience and safety without compromising efficacy[7]. The combination of cisplatin and capecitabine showed an overall response rate of 46%-54.8% in advanced gastric cancer[8,9]. In addition, in a phase II study using preoperative docetaxel, capecitabine and cisplatin in initially locally advanced unresectable gastric cancer a R0 resection could still be achieved in 63% of the patients with an acceptable toxicity (febrile neutropenia 4%, no treatment related mortality)[10].
Taking these promising results into consideration we decided to conduct a one arm phase II trial investigating the feasibility of 4 cycles of preoperative chemotherapy with docetaxel, cisplatin and capecitabine in patients with resectable gastric cancer, followed by a standardized gastric resection and lymphadenectomy.
Inclusion criteria were histologically proven gastric cancer [including gastro-oesophageal junction/cardia carcinoma (Siewert 2 and 3[11])], stage Ib-IVa (6th TNM classification), WHO performance state 0-1, age ≥ 18 years and adequate hematologic, renal and hepatic function. All patients signed an informed consent and were expected to comply with treatment, management of toxicity and scheduled follow-up. Exclusion criteria were non-resectability, previous or current malignancies, other serious illness or medical conditions, known hypersensitivity to any of the chemotherapies used, contraindication for the use of corticosteroids, use of immunosuppressive or antiviral medication, and pregnant or lactating women. A certified ethics committee (METOPP) and the institutional review board at each centre approved the protocol. Screening included a history and physical examination, structural assessment of malnutrition, oesophagoduodenoscopy, blood sampling and CT scan of the chest and abdomen. Evaluation CT-scans were performed after the second and fourth cycle of chemotherapy.
Chemotherapy: Preoperative chemotherapy was administered for four cycles. Based on the described by Sym et al[10], each 3-wk cycle consisted of docetaxel 60 mg/m2 IV infusion and cisplatin 60 mg/m2 IV infusion on day 1, and capecitabine 1.875 mg/m2 orally on days 1-14 divided into two daily doses (DCC). Prior to each cycle a full physical examination was performed, and a full blood count and chemistry was obtained. The neutrophil count had to be ≥ 1.5 × 109/L and the platelet count ≥ 100 × 109/L. Dose reductions and delays were predefined for granylocytopenia, thrombocytopenia, and non-hematological toxicity. Secondary use of growth factors was not part of the protocol. Any adverse event was collected and registered according to Common Toxicity Criteria (CTC, version 3). A serious adverse event (SAE), defined as an event that is either fatal, life-threatening, requiring or prolonging hospitalization or resulting in persistent or significant disability or incapacity, was reported to the study coordination centre, and evaluated by the principle investigators. Furthermore, these SAE’s were reported to the central medical ethics committee.
Surgery and pathology: Patients were scheduled for surgery approximately four to six weeks after the last cycle of chemotherapy. A (partial) gastric resection and a standardized lymphadenectomy, the so-called D1extra lymphadenectomy specified to tumour location was performed by a local surgeon specialized in gastrointestinal surgery, assisted by a surgeon of the study team. The D1extra lymphadenectomy is a newly defined dissection in which lymph node stations 1-10 and/or 12 (according to the Japanese Classification[12]) prone to metastases[13] are removed.
The primary endpoint of this feasibility study was the toxicity and safety profile of 4 courses of DCC in patients diagnosed with primary resectable gastric cancer. The secondary endpoint of this study was the determination of pathological complete response (pCR) and pathological resection rate (R0). The results, e.g., numbers and proportions of patients reaching the primary and secondary endpoints, will be evaluated using describing statistical analyses.
Between November 2008 and November 2012, 53 patients from five participating hospitals were included in the study. Two patients were classified by the monitoring committee as having distal oesophageal cancer instead of gastric cancer and were therefore excluded from the study. In Table 1 the patient characteristics are outlined. The median age was 64 years (range 34-84), and 75% of the patients exhibited an WHO performance state of 0. One patient having a WHO performance state of 2, as re-assessed later on, was not excluded because of an intention-to-treat protocol.
| Characteristics | No. of patients | % |
| Age, yr | ||
| Median | 64 | |
| Range | 34-84 | |
| Age, category | ||
| < 50 yr | 5 | 9.8 |
| 50-59 yr | 8 | 15.7 |
| 60-69 yr | 22 | 43.1 |
| 70-79 yr | 15 | 29.4 |
| > 80 yr | 1 | 2 |
| Sex | ||
| Male | 36 | 70.6 |
| Female | 15 | 29.4 |
| WHO performance status1 | ||
| 0 | 37 | 72.5 |
| 1 | 13 | 25.5 |
| 2 | 1 | 2 |
| Clinical T stage2 | ||
| T1 | 5 | 9.8 |
| T2 | 12 | 23.5 |
| T3 | 21 | 41.2 |
| T4 | 2 | 3.9 |
| Unknown | 11 | 21.6 |
| Clinical N stage2 | ||
| N0 | 16 | 31.4 |
| N1 | 19 | 37.3 |
| N2 | 4 | 7.8 |
| N3 | 2 | 3.9 |
| Unknown | 10 | 19.6 |
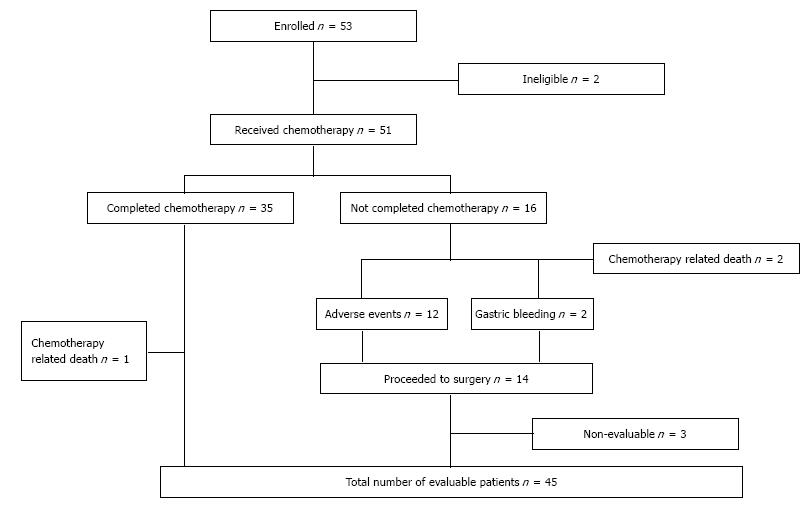
All 51 patient started preoperative chemotherapy. In total, 35 patients completed 4 cycles of chemotherapy (68.6%). In Table 2 the feasibility results are outlined. A total of 169 cycles of chemotherapy were administered. The percentage of intended dose delivered in the intention-to-treat group was 78%-79% for each drug, calculated as the percentage of dose delivered in patients eligible for chemotherapy (deceased patients were excluded). Reasons for dose reduction and discontinuation were treatment related toxicity, including two deaths and a tumour related bleeding in two patients (Figure 1).
| No. of patients | % | |
| Cycles received | ||
| 1 | 51 | 100 |
| 2 | 44 | 86.3 |
| 3 | 39 | 76.5 |
| 4 | 35 | 68.6 |
| Percentage of intended dose delivered (per evaluable patient, ITT)1 | ||
| Docetaxel | 78.90 | |
| Cisplatin | 78.70 | |
| Capecitabine | 78.30 | |
| Percentage of intended dose delivered in patients receiving 4 courses (n = 34) | ||
| Docetaxel | 92.90 | |
| Cisplatin | 92.90 | |
| Capecitabine | 91.60 |
All patients were evaluable for safety. Grade III/IV toxicity is summarized in Table 3. The most common grade III/IV toxicity was febrile neutropenia and diarrhea occurring in 10.1% and 9.5% of the cycles, in respectively 31% and 25% of patients. There were 3 chemotherapy related deaths, resulting in a mortality rate of 5.9%. In two patients, treatment-related death was infection concomitant with grade III/IV neutropenia. One patient died after refusing further therapy of an initially successful treatment of febrile neutropenia.
| Toxicity | No of patients | % | No of cycles | % |
| Hematologic | ||||
| Anemia | 3 | 5.9 | 3 | 1.8 |
| Neutropenia | 25 | 49 | 32 | 18.9 |
| Febrile neutropenia | 16 | 31.4 | 17 | 10.1 |
| Non-Hematologic | ||||
| Gastro-intestinal | ||||
| Anorexie | 8 | 15.7 | 10 | 5.9 |
| Constipation | 1 | 2 | 1 | 0.6 |
| Diarrhea | 13 | 25.5 | 16 | 9.5 |
| Dysphagia | 1 | 2 | 1 | 0.6 |
| Mucositis | 6 | 11.8 | 6 | 3.6 |
| Nausea | 5 | 9.8 | 5 | 2.9 |
| Vomiting | 5 | 9.8 | 8 | 4.7 |
| Constitutional | ||||
| Fatigue | 4 | 7.8 | 4 | 2.4 |
| Hand-foot syndrome | 4 | 7.8 | 6 | 3.6 |
| Neurosensory | ||||
| Hearing impairment | 1 | 2 | 1 | 0.6 |
| Neuropathy | 2 | 3.6 | 2 | 1.2 |
| Renal impairment | 3 | 5.9 | 3 | 1.8 |
Of the remaining 48 patients, 3 patients were considered non-evaluable for the secondary endpoints because of major protocol violation (one patient was operated one year after completion of the preoperative regimen due to myocardial infarction, one patient switched to another chemotherapy regimen, and one patient was operated in a non-participating hospital). Of the remaining 45 patients 39 patients underwent a R0 resection. Two patients developed distant metastases assessed prior to surgery, two patients had peritoneal carcinomatosis diagnosed during explorative surgery and two patients had a R1 resection. Thus, 76.5% of the intention to treat population and 86.7% of the evaluable patients had a R0 resection with curative intend. The surgical results are described elsewhere. A pCR was reported in 3 patients (5.9%).
Overall survival of gastric cancer after a curative resection can be improved with perioperative chemotherapy as shown in the MAGIC trial. The additional benefit of perioperative ECF on survival is probably for the larger part attributed to the preoperative part of the treatment[3]. Postoperative chemotherapy in this patient category is challenging since a high percentage of the patients is not fit enough or willing to start and complete the full postoperative part of the regimen[3]. To improve the adherence and increase the benefit of preoperative chemotherapy in resectable gastric cancer we designed this phase II study investigating the feasibility of a preoperative regimen of four cycles of docetaxel, cisplatin and capecitabine. To increase the efficacy of the preoperative regimen, we replaced epirubicin by docetaxel, since docetaxel containing combination regimens have shown to be feasible and have good response rates in locally-advanced and metastatic gastric cancer[4-6]. In our trial however, four courses of DCC as a preoperative regimen showed to be highly demanding for patients with primarily resectable gastric cancer. Only sixty-eight percent of the patients completed all 4 cycles of DCC, the other patients discontinued mainly due to treatment related toxicity. In comparison with results from other trials this percentage is rather low. In a German phase II trial investigating the same regimen as perioperative chemotherapy, with a higher dosage of docetaxel of 75 mg/m2, 94% completed all three preoperative cycles[14]. In the MAGIC trial, 86% completed the intended three preoperative cycles of ECF[3]. In a French trial the rate of patients completing two cycles of preoperative chemotherapy was 87%[15], while in an Italian study the rate of completing 4 preoperative docetaxel based cycles was 74%[16]. Four cycles of preoperative DCC chemotherapy, therefore, might be too demanding whereas 86% and 76% of the patients in our study completed 2 and 3 cycles respectively which is comparable to the results described above. On the other hand, completing postoperative chemotherapy is even more difficult. In the aforementioned Italian study feasibility of preoperative chemotherapy was compared to the feasibility of the same regimen as postoperative chemotherapy. The rate of completing 4 postoperative cycles was 34% in this arm[16]. In the previous mentioned German and MAGIC trials only 53% and 42% respectively completed the postoperative scheme[3,14]. Although the rate of completing all 4 cycles was relatively low in our study, the intended delivered dose was reasonable with percentages of 78 for all drugs individually[7,14]. Accurate monitoring and early intervention in case of deterioration is imperative to prevent a high amount of patients failing to complete a full chemotherapy regimen.
Treatment related mortality was 5.9% being comparable to mortality rates reported in literature (0%-6%)[4,5,7,17]. Febrile neutropenia occurred in 10% of all cycles (vs 2%-15% found in other trials[4,5]), being the cause of death of at least two of three patients. The prophylactic or secondary use of G-CSF was not part of the protocol as no data were available at the time of the study design about the interaction between G-CSF and capecitabine in case of simultaneous administration. In theory, the proliferative activity of bone marrow after the administration of G-CSF might increase the myelotoxicity of capecitabine. In literature, only scarce data are known about the simultaneous use of G-CSF and capecitabine. In a phase II trial in breast cancer, the use of pelfilgastrim was evaluated in a small subset of patients receiving docetaxel and capecitabine based chemotherapy regimen. Minimal grade III/IV neutropenia and no febrile neutropenia was observed[18]. In one phase II trial in metastatic gastric cancer with a comparable DCC regimen as in our study, patients were treated successfully with G-CSF in case of febrile neutropenia and no toxicity related deaths were reported[19]. The use of G-CSF as primary or secondary prophylaxis for (febrile) neutropenia in a docetaxel and capecitabine based chemotherapy scheme is therefore promising, and should be further investigated.
Other main toxicities we encountered were grade III/IV hand-foot syndrome, diarrhea and anorexia. The rate of hand-foot syndrome of 7.8% in this study is acceptable compared to other studies[7-10,17]. Many patients with gastric cancer experience difficulties with eating. With addition of the toxicity of chemotherapy gastric cancer patients are prone to anorexia and weight loss. It is therefore imperative to monitor their intake and weight to be able to act in time when this is deteriorating. A dietician should be consulted and enteral feeding should be started in an early phase[20].
In gastric cancer, clinical tumour staging faces several difficulties. The current imaging modalities have low sensitivity rates for T- and N-stage[21]. It is therefore difficult to clinically assess the efficacy of chemotherapy in these patients. In literature, many modalities have been used to determine response rate[4,7,15], which makes it difficult to compare ORRs. In our study, we therefore only determined pathological response rate. A pCR was found in 3 patients (5.9%) which is lower than expected looking at other studies investigating DCF or DCC in gastric cancer in which pCRs of 6.1%[10], 11.7%[16] and 13.7%[14] are reported. On the other hand, in the MAGIC trial using ECF as a treatment regimen no pCR was seen[3].
Thirty-nine (76.5%) patients received a R0 resection. This is in line with rates found in the MAGIC trial (69.3%)[3], although it is lower compared to other trials using a docetaxel based regimen in which a R0 resection was achieved in 84%[15], 85%[16], and 90.2%[14] of patients. The long-term effects of this docetaxel based scheme and protocolized D1extra lymphadenectomy have to be awaited.
In conclusion, in our study the benefits defined as R0 resection and complete pathological response rates of four cycles of DCC are lower than expected, although the effects on long-term results have to be awaited. Moreover, this is coupled with a high percentage of grade III/IV toxicity, especially febrile neutropenia. The use of simultaneous G-CSF and capecitabine should be further investigated to decrease toxicity-related non-adherence and mortality. According to the results of this study, the use of DCC without G-CSF support as preoperative regimen in resectable gastric cancer is debatable.
We would like to acknowledge Sanofi for their support in this study.
Survival rates for resectable gastric cancer are still poor. Resection is the cornerstone of treatment, though the addition of perioperative chemotherapy has additional benefit. In 2006, the results of the MAGIC trial were published, comparing perioperative chemotherapy with surgery alone, which resulted in a survival benefit. Only 42% of patients completed the postoperative regimen consisting of epirubicine, cisplatin and capecitabine. Other regimens have been investigated for their effectiveness in gastric cancer, e.g., docetaxel combined with cisplatin and capecitabine, leading to promising results.
Improve survival of curable gastric cancer with the use of different regimens of (neo)adjuvant chemotherapy.
At the time of study design, this was one of the first phase II studies to investigate the feasibility of a docetaxel based regimen in resectable gastric cancer. Although the R0 resection rates were high, it was accompanied by a high rate of febrile neutropenia which resulted in a mortality rate of 5.9%.
The combination of docetaxel, cisplatin and capecitabine could be used as a (neo)adjuvant regimen in the setting of resectable gastric cancer, although the role of granulocyte colony-stimulating factor (G-CSF) to prevent febrile neutropenia should be investigated.
Docetaxel can cause neutropenia. In case of an infection, this can be fatal complication. G-CSF could prevent the development of neutropenia, thereby preventing this major complication.
The present study is a phase II clinical trial which had the aim to evaluate the feasibility of three-drug regimen of preoperative chemotherapy of gastric cancer, composed by cisplatin, capecitabine and docetaxel. It is a well-conducted study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jacome AAA, Rakusic Z, Song HS S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3657] [Article Influence: 304.8] [Reference Citation Analysis (2)] |
| 2. | Dassen AE, Dikken JL, Bosscha K, Wouters MW, Cats A, van de Velde CJ, Coebergh JW, Lemmens VE. Gastric cancer: decreasing incidence but stable survival in the Netherlands. Acta Oncol. 2014;53:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 4. | Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, Köberle D, Borner MM, Rufibach K, Maibach R. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S, Van Cutsem E. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660-5667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1458] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 7. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1693] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 8. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 9. | Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, Kim BS, Lee JS. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol. 2002;13:1893-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, Oh ST, Kim BS, Kang YK. Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol. 2010;17:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 914] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 960] [Reference Citation Analysis (0)] |
| 13. | Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Ono H, Akiyama H. Distribution of lymph node metastasis in gastric carcinoma. Hepatogastroenterology. 2006;53:468-472. [PubMed] |
| 14. | Thuss-Patience PC, Hofheinz RD, Arnold D, Florschütz A, Daum S, Kretzschmar A, Mantovani-Löffler L, Bichev D, Breithaupt K, Kneba M. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO){dagger}. Ann Oncol. 2012;23:2827-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 16. | Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 17. | Thuss-Patience PC, Kretzschmar A, Dogan Y, Rothmann F, Blau I, Schwaner I, Breithaupt K, Bichev D, Grothoff M, Grieser C. Docetaxel and capecitabine for advanced gastric cancer: investigating dose-dependent efficacy in two patient cohorts. Br J Cancer. 2011;105:505-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wenzel C, Bartsch R, Locker GJ, Hussian D, Pluschnig U, Sevelda U, Gnant MF, Jakesz R, Zielinski CC, Steger GG. Preoperative chemotherapy with epidoxorubicin, docetaxel and capecitabine plus pegfilgrastim in patients with primary breast cancer. Anticancer Drugs. 2005;16:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Polyzos A, Felekouras E, Karatzas T, Griniatsos J, Dimitroulis D, Polyzos K, Kontzoglou K, Mantas D, Karavokyros J, Nikiteas N. Modified docetaxel-cisplatin in combination with capecitabine as first-line treatment in metastatic gastric cancer. a phase II study. Anticancer Res. 2012;32:4151-4156. [PubMed] |
| 20. | Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, Selga G, van Bokhorst-de van der Schueren MA, von Meyenfeldt M, Zürcher G, Fietkau R. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr. 2006;25:245-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E, Tinmouth J. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |