Published online Oct 27, 2016. doi: 10.4240/wjgs.v8.i10.660
Peer-review started: June 18, 2016
First decision: July 27, 2016
Revised: August 14, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: October 27, 2016
Processing time: 130 Days and 10.7 Hours
Malignant neoplasms of the appendix are rare and represent less than 1% of gastrointestinal cancers. Goblet cell carcinoids (GCC) tumors are a distinctive group of heterogeneous appendiceal neoplasm that exhibit unique clinical and pathologic features. This review focuses on the current diagnostic procedures, pathogenesis, possible signaling mechanisms and treatment options for GCC. Perspectives for future research are discussed. The tumor likely arises from pluripotent intestinal epithelial crypt base stem cells. Previous findings of Notch signaling as a tumor suppressor in Neuroendocrine tumors may have a similar role in this tumor too. Loss of Notch signaling may be the driver mutation with other successive downstream mutations likely favors them into progressing and behavior similar to poorly differentiated adenocarcinoma with minimal neuroendocrine differentiation. A multidisciplinary approach is suggested for optimal outcomes. Surgery remains the main treatment modality. Simple appendectomy may be sufficient in early stages while right hemicolectomy is recommended for advanced tumors. Cytoreductive surgery with heated intraperitoneal chemotherapy may improve survival in a select few with metastatic peritoneal disease. These tumors have an unpredictable behavior even in early stages and local recurrence and delayed metastases may be seen. Lifelong surveillance is warranted.
Core tip: Goblet cell carcinoids tumors are a distinctive group of heterogeneous appendiceal neoplasm that exhibit unique clinical and pathologic features. The pathogenesis is unclear however the tumor likely arises from pluripotent intestinal epithelial crypt base stem cells. Loss of Notch signaling may be the driver mutation with other successive downstream mutations likely favors them into progressing and behavior similar to poorly differentiated adenocarcinoma with minimal neuroendocrine differentiation. Surgery remains the main treatment modality. We discuss the clinical implications of this cancer focusing on the tumor biology, mutations, signaling mechanisms and management.
- Citation: Shenoy S. Goblet cell carcinoids of the appendix: Tumor biology, mutations and management strategies. World J Gastrointest Surg 2016; 8(10): 660-669
- URL: https://www.wjgnet.com/1948-9366/full/v8/i10/660.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i10.660
Malignant neoplasms of the appendix are rare and represent less than 1% of gastrointestinal cancers. Studies evaluating data for appendiceal malignancies from seer database between 1973-2001 showed the age-adjusted incidence of cancer of appendix was 0.12 cases per 1 million people per year[1,2]. They are further classified into colonic type adenocarcinoma, mucinous tumors, signet ring cell tumors, carcinoids [neuroendocrine tumors (NETs)] and goblet cell carcinoids (GCC). Overall five-year survival is highest for appendiceal carcinoid (83%) and lowest for signet ring cancers (18%)[1,2]. This review focuses on GCC of the appendix. The current diagnostic procedures, pathogenesis, signaling mechanisms and possible mutations are presented. Treatment options for this neoplasm are defined and summarized, although evidence-based data are lacking. Surgery remains the treatment mainstay.
GCC tumors are a distinctive group of heterogeneous appendiceal neoplasm that exhibit unique clinical and pathologic features. These hybrid tumors have both glandular and neuroendocrine morphology and are designated with various terminologies: Adenocarcinoids, crypt cell carcinoma, mixed carcinoid-adenocarcinoma and amphicrine tumors. These various terminologies do not reflect consistent morphology, biologic behavior or accepted criteria for the diagnosis. GCC was first described by Gagné et al[3] in 1969, Subbuswamy et al[4] subsequently coined the term GCC in 1974. Warner et al[5] in 1979 suggested a probable origin from crypt based stem cell. Isaacson et al[6] in 1981 demonstrated presence of IgA, lysozyme in GCC suggestive of possible role of Paneth cells in this tumor[3-6].
GCC exhibits clear distinction when compared to appendiceal NETs or primary adenocarcinoma in terms of demographics, biology and clinical aggressive behavior. The prognoses of GCC lays intermediate between appendiceal NETs and primary appendiceal adenocarcinoma[1,2].
GCC are diagnosed in less than 1% of appendectomy specimens[7,8]. Most commonly, patients present with abdominal pain and acute appendicitis (> 50%). They are most often diagnosed incidentally during appendectomy or ileocecal resection and confirmed by the pathologist in post-surgical specimens. About 27% of patients may present with perforated appendicitis[9]. Patients may also present sub-acutely in advanced stages with vague abdominal pain and mass[7,8]. Common in Caucasians, there is equal distribution in male and females with the average age of diagnosis in the fifth decade[1,2]. Up to 50% of patients present with metastatic disease[8,10-12]. Similar to carcinoids a significant number of these patients may harbor a second primary malignancy[12-14].
Morphologically the tumor circumferentially involves the appendiceal wall with transmural extension. Submucosal involvement with mucosal sparing is noted. Most tumors are generally > 2 cm in size. The native appendiceal epithelium may show fibrous obliteration without adenomatous or dysplastic changes[8,15].
GCC display a wide range of histologic patterns both in primary and metastatic sites. Common to all GCC is the presence of mucin containing goblet shaped epithelial cells arranged in clusters in the lamina propria and submucosa. These cells stain positive for mucicarmine, periodic acid–Schiff and alcian blue stains suggestive of goblet cell mucin. Extracellular pools of mucin may also be present. Also seen are cells, which demonstrate focal, inconsistent scattered immunoreactivity for neuroendocrine markers (i.e., chromogranin, synaptophysin)[8,16].
Since GCCs show a submucosal growth pattern, it has a tendency to spread to surrounding bowel. The most common metastatic sites include direct extension into the right colon and ileum, followed by spread to lymph nodes, peritoneum and omentum. The ovaries are common site of metastases in women presenting as Krukenberg tumor. Up to 80 % of women with stage 4 disease present with ovarian metastases[8,12]. Solid organ metastases to liver, lung, bones are uncommon[8]. A previous study reports the rate of metastases to lymph nodes increases with the T stage of the tumor T2 (0%), T3 (13%), T4 (60%)[9].
Metastatic lesions from GCC are more aggressive tumors and often show poorly differentiated signet ring cell or undifferentiated adenocarcinoma morphology with minimal neuroendocrine features. They may not share features of primary tumor and carry poorer prognosis. Metastatic tumors usually do not stain for chromogranin A or synaptophysin and stain heavily for mucin, suggestive of degeneration into signet ring cell morphology. The population of endocrine cells and Paneth cells seem to decrease in metastatic lesions. Yan et al[17] in their series of 26 patients reported that nine patients (35%) with metastatic GCC failed to stain for neuroendocrine marker. The explanation for this finding remains elusive[17-21].
Currently multiple classification systems exist to describe GCC.
The 2010 World Health Organization (WHO) classification for tumors of the appendix, classifies GCC under the category of neuroendocrine neoplasms based on differentiation and histological grading. Grade refers to the proliferative activity measured with mitotic counts and Ki-67 index. They are further sub-classified as low grade: G1 (< 2 mitosis/10 HPF and ≤ 2% Ki index), intermediate grade: G2 (2-20 mitosis/10 HPF, 3%-20% Ki index) and high grade: G3 (> 20 mitosis/10 HPF, > 20% Ki index). Differentiation refers to resemblance of tumor cells to the normal neuroendocrine cells. Carcinoids (well differentiated neuroendocrine neoplasm) generally belong to G1 and G2 categories while G3 is considered as a neuroendocrine carcinoma (NEC). Goblet cell tumors are subtyped under mixed adeno-NEC (MANEC). To qualify for this definition at least 30% of tumor should have gland forming epithelial and neuroendocrine components[22].
The 2010 American joint commission on cancer (TNM classification) stages these tumors based on the tumor size, nodal status and metastatic disease into stages (I-IV). Stage I (T1, N0, M0), stage II (T2/T3, N0, M0), stage III (any T/N1, M0), and stage IV (any T /any N/M1)[23].
Tang et al[8] in 2008, proposed a system of classification specific for GCC of appendix based on histologic features of the tumor at the primary site. They include the arrangement of the goblet cells, degree of atypia and desmoplasia to label these tumors into three groups. Typical GCC (group A); adenocarcinoma ex GCC, signet ring cell (group B); adenocarcinoma ex GCC, poorly differentiated (group C). Almost all patients in group C presented in advanced stages with wide metastases. This suggests that GCCs display a spectrum of histologic features with the potential to progress to an aggressive adenocarcinoma phenotype[8].
These multiple pathologic definitions and differing terminologies have led to inconsistent reporting and difficult to characterize this disease.
MANEC per the 2010 WHO classification are tumors harboring both epithelial and neuroendocrine components. However based on this definition it requires tumors to have at least 30% representation of each component. In general this is not true for all GCC tumors. Further advanced stages of GCC losses its neuroendocrine differentiation and acquires an aggressive signet ring cell or poorly differentiated morphology.
This tumor may need further investigations to better clarify and define their heterogeneous, molecular profile and classification.
GCC specimens demonstrate focal, inconsistent immunoreactivity for neuroendocrine markers. In contrast diffuse staining is observed in most classic carcinoids of the appendix. Common positive markers are synaptophysin, chromogranin A, serotonin, neuron specific enolase, pancreatic polypeptide. Ultrastructural immuno- histiochemistry staining has shown tumor nests resembling normal crypts in the submucosa. Separate goblet cell and neuroendocrine cells are often located in close proximity to each other[24] (Table 1).
| Markers | Goblet cell carcinoid | Typical carcinoid | Adenocarcinoma |
| CEA | + | - | + |
| CK7 | + | - | + |
| CK20 | + | - | + |
| CDX2 | + | - | + |
| CD56 | +/- | ++ | - |
| CAM5.2 | + | - | + |
| Synaptophysin | +/- | ++ | - |
| Chromogranin A | +/- | ++ | - |
| Β-Catenin (nuclear) | - | - | + |
| p53 | +/- | - | ++ |
| Ki67% | +/- | +/- | ++ |
| MUC1 | - | - | ++ |
| MUC2 | ++ | - | +/- |
| E-cadherin expression | N | N | U |
| MATH-1 expression | + | + | - |
| KRAS mutation | - | - | + |
| SMAD4 mutation | - | - | + |
| Notch signaling inhibition | U | + | - |
| MMR (MSH2, MSH6, MLH1, PMS2) | - | - | +/- |
In addition GCC do not exhibit mutations as conventional colorectal adenocarcinoma. These tumors are negative for KRAS, SMAD4 and BRAF mutations[25]. They show negative staining for nuclear β-catenin and for p53. MUC2 expression is preserved[8]. Normal colorectal and appendicular epithelium expresses MUC2 only. GCC show strong carcinoembryonic antigen, caudal type homeobox transcription factor 2, cytokeratin 7 (CK7), CK20 expression suggestive of intestinal epithelial origin while these markers remain negative in classic carcinoids[26]. A single study showed allelic loss in chromosomes 11q, 16q, and 18q in GCC similar to ileal carcinoids[25].
The proliferative Ki67 index remains low in typical GCC but rises with advanced stages (Tangs group C). The significance remains unknown as some groups have shown worsening survival rates with rising Ki67 index[19,27] while other have shown no correlation[9,28,29]. Positive staining for p53 and MUC1 with loss of MUC2 expression is suggestive of transformation to adenocarcinoma phenotype similar to colorectal adenocarcinoma[8]. This also correlates with the rising Ki67 index as reported with Tangs et al[8]’s classification.
In general patients with GCC do not present with carcinoid syndrome and urinary 5HIAA levels are within normal range[16]. Unlike classic midgut carcinoids, serum chromogranin A levels are normal and have no value in detecting and monitoring GCC. Somatostatin expression is sparse and erratic and therefore functional scans such as 111-Indium pentetreotide scintigraphy (Octreoscan) and Gallium 68-octreotide positron emission tomography (PET) scans are usually normal in patients with GCC, and thus are of limited use[11,19]. Fluorodeoxyglucose PET scan may be useful in advanced disease to detect peritoneal metastatic disease[7,27,30].
GCC also express transcription factor Math-1 and HD5 (Defensins) a known marker for Paneth cells[26]. Math-1 is a basic helix-loop-helix transcription factor essential for development of the pluripotent stem cell towards secretory stem cell lineage and may play a role in pathogenesis of GCC.
The pathogenesis of GCC remains unclear. Unlike adenocarcinomas of the GI tract which arises through an adenoma-carcinoma sequence GCC is thought to arise from pluripotent intestinal epithelial crypt base stem cells[6,26].
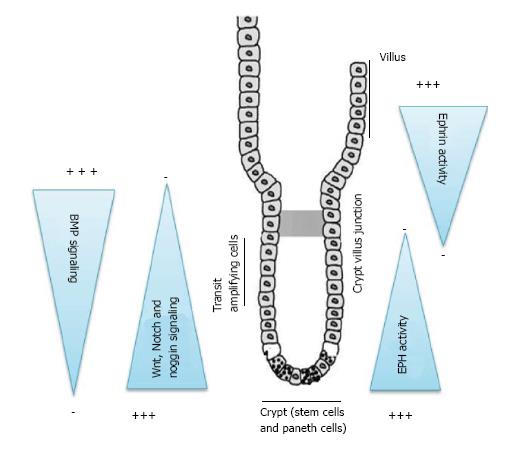
An understanding of the embryological origin and signaling pathways associated with development of small bowel and appendix may provide clues and explain the origin and progression of GCC. The epithelial lining of small gut consists of a single layer of columnar cells. This differentiated epithelium arises from the crypts and projects up as villi into the lumen forming the absorptive lining of the gut (Figure 1). Villi begin to form by embryonic day 15 and crypt form by invagination of intervillus pockets at post-natal day 7[31,32]. The four main types of differentiated cells are absorptive enterocytes, goblet cells, neuroendocrine and Paneth cells. The crypt thus forms the proliferative stem cell compartment, where these cells originate, differentiate, amplify and move up into the villi akin to a system of conveyor belt in an assembly line[33]. Paneth cells which also originate from the crypt in an exception and migrates downward into the base of the crypt. These four cell types are the main differentiated cell types found in the epithelial lining of the small intestine.
How is the stem cell niche created and defined? What molecular signaling mechanisms keep the niche intact and regulated? Information on these fundamental questions comes from mouse studies. Advances in development and stem cell biology have also occurred to generate complex three dimensional human intestinal tissues in vitro through directed differentiation of human pluripotent stem cells. These human intestinal organoids called mini-gut have expanded our ability to study development, genetics, intestinal pathogens and metabolic disease and cancer[34]. Cheng and Leblond[35,36] in 1974 were first to characterize crypt based columnar cells as intestinal stem cells. Barker et al[37] identified a marker Lgr5/GPR49, a leucine rich orphan G-protein coupled receptor that labels these stem cells. Tritiated radioactive thymidine labelling experiments have confirmed that these Lgr5 cells are the multipotent stem cells. Stem cell division occurs every 24 h and these cells are localized to the crypts. Subsequently cells migrate up from the crypt to the villus in 3-5 d[34,38]. These stem cells by itself are not terminally differentiated and can divide without a limit. The daughter cells have to choose between committing to terminal differentiation or remain as a stem cell. The rapidly dividing groups of cells derived from the crypt stem cells that have committed to differentiation are known as transit amplifying cells. As they migrate further up the crypt they amplify according to their prospective destined fate and differentiate as enterocyte, goblet cell, neuroendocrine and Paneth cell. They cease to divide further once they reach the neck of the crypt at the crypt villous junction[31,33].
Thus this slim, columnar crypt based Lgr5 positive stem cells along with the post mitotic Paneth cells form the stem cell niche through which begins the growth and renewal of all the differentiated cells of the small intestinal epithelium.
There are two major groups of cell signaling mechanisms which govern the crypt-villus axis (Figure 1). The first is an epithelial-epithelial cell communication. The key mediators of these mechanisms are Wnt, Notch, Eph-ephrin and Math 1 signaling pathways. Together and sequentially they are primarily responsible for maintaining the gut stem cells in a proliferative state, differentiation into secretory or absorptive lineage and establish boundaries between these clones of cells. Mutation in these critical pathways has been implicated with excessive uncontrolled, ectopic crypt formation, adenomas, excessive goblet cell or neuroendocrine cells and other risks for colorectal malignancies.
The second major group of cellular signaling comprises of epithelial-mesenchymal (EMT) communications and the mediators in these pathways consist of hedgehog, BMP and PDGAF signaling pathways. The essential function of EMT pathways is to maintain a proper spacing between one crypt and the next. They are negative regulators of crypt formation. Hedgehog signaling increases the expression of BMP in the mesenchyme which further represses the Wnt signaling. Noggin, a BMP inhibitor is expressed in the crypts to maintain unsuppressed Wnt activity in the crypt epithelium (Figure 1). Mutation in these pathways or inhibition of BMP signaling by overexpression of its inhibitors, Noggin or inactivation of its receptor BMPRIA lead to excessive and ectopic crypt formation as seen with juvenile polyposis syndrome due to BMP knock out mutations[39].
We will limit our discussion to the epithelial-epithelial signaling mechanisms which may hold clues to the pathogenesis of GCC.
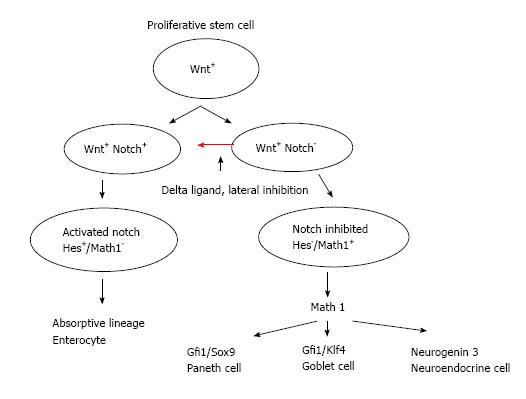
The initial signaling mechanisms in a crypt-villus axis begin with Wnt and Notch pathways in the crypts (Figure 1). Both the development of small intestine and its homeostasis require canonical Wnt signaling. The Wnt pathways maintain the gut stem cell compartment. The Paneth cell which constitutes part of the stem cell niche generates Wnt signals that act over a short range and keep cells in the crypt in a proliferative state[31,33]. There exists a gradient in Wnt signaling which is highest in the crypt base and diminishes towards the crypt-villus junction (Figure 1). Wnt signaling further drives the expression of Notch pathway. Notch pathway through its ligands such as Delta and jagged and effectors such as Hes and NF-κB transcription factors mediate lateral inhibition within the Wnt activated cell population thus driving cells towards different fates. Delta expressing cells escape Notch activation (Wnt+, Notch-) and commit to secretory fate through the downstream Math-1 signaling pathways and exit into a committed fate[32,33,40]. Meanwhile (Wnt+/Notch+) cells continue to migrate up the crypt and divide, generating daughter cells and diversify till they lose Wnt activation as they move up the villus and differentiate as absorptive enterocytes. Inactivation of Notch pathway by deletion of Hes 1 or nonsense mutation of Delta ligands leads to excessive formation of goblet cells and neuroendocrine cells[32,33,40]. Further studies have shown that inhibiting transcription factors in the Notch pathways by deletion of RBP-jk or by use of γ-secretase inhibitors prevents proteolytic cleavage and the release of notch intracellular domain complex. This result in epithelial cells composed exclusive of goblet cells[41]. Conversely experiments have shown that increased Notch activity results in severe reduction of differentiated secretory cells, suggesting a tumor suppressor role of Notch signaling in neuroendocrine tumors[42].
In addition to Wnt-Notch signaling another significant pathway in the crypt-villus axis known to play a role in maintaining cellular boundaries, segregation and establish migratory path are the Eph-ephrin molecules (Figure 1). Expression of Eph B receptors and the Ephrin-B ligands within the intestine is regulated via the β-catenin-TCF transcription complex through the Wnt pathway. A definitive gradient exists with proliferative cells in the crypt expressing higher density of Eph-B receptors and progressively decreases at the crypt villus axis. The cells acquiring a differentiated fate switch off the expression of Eph-B and switch on the expression of Ephrin B ligands which progressively increases as they migrate up the axis. Paneth cells express only Eph receptors and therefore remain at the crypt base. In general cells expressing Eph receptors are repelled by contact with cells expressing ephrins on their surface. These mechanisms keep the cells segregated in their respective niches. Dysregulation of the eph-ephrin axis leads to cellular derangement with proliferating cells are not restricted to the bottom of the crypt and abnormally scattered along the crypt-villus axis[33]. Eph B mediated compartmentalization restricts the spreading of Eph B expressing tumor cells into ephrin B1-positive territories in vitro and in vivo. Loss of EphB-mediated compartmentalization may lead to invasiveness of the tumor cells[43].
All three secretory cell types derive from a precursor expressing Math-1 (mouse atonal homologue 1), also known as Atoh1, Hath-1 (humans) (Figure 2). As explained earlier Delta expressing cells escape Notch activation (Wnt+, Notch-) and commit to secretory fate through the downstream Math-1 signaling pathways[32,33,40]. It is a basic helix-loop-helix transcription factor that is required for secretory cell lineage through downstream Neurogenin 3 for neuroendocrine cells, Gfil and Klf4 for goblet cells, β-catenin and sox9 for Paneth cells and subsequent cell cycle exit[31,32,44,45]. Mice deficient in Math1 lack goblet cells and the epithelial cells continue to maintain their proliferative state[32,40]. Overexpression of Math1 results in ectopic secretory cells[46]. The immunohistochemical expression of Math-1 in GCC suggests that this transcription factor is essential for normal development of the pluripotent stem cell towards secretory stem cell lineage and may play a role in its pathogenesis. Possible somewhere along its differentiation a mutation occurs with altered signaling pathways which causes excessive clones of goblet cells and neuroendocrine cells and may explain the hybrid nature of this tumor[18,26].
NETs including GCC appear to be heterogeneous group of tumors with varying signaling mechanisms and gene expressions in different tissue of origin. A number of questions remain to be answered. Is there a Notch signaling dysfunction or inhibition leading to loss of Hes regulated inhibition of Math-1? Studies have confirmed the potential oncogenic role of Notch signaling and its transcription factor in certain solid organ abdominal, lung, breast, and genitourinary, neural and hematological malignancies[47]. However Notch signaling also appears to have a tumor suppressor role in gastrointestinal, thyroid and pulmonary neuroendocrine tumors[42,48,49]. In another recent study of 31 ileal carcinoids, Notch signaling was uniformly absent in ileal neuroendocrine tumors suggestive of loss of tumor suppressive role[50]. Is the loss of Notch signaling, the driving mutation and occurs after the first stem cell division at the level of transit amplifying cells with subsequent progeny showing dysfunction? Could there be a concurrent Eph-ephrin pathway mutation along with loss of notch signaling, leading to loss of compartmentalization of cells and portending invasiveness[43]?
Why does metastatic GCC show minimal neuroendocrine expression and more of signet ring cell and poorly differentiated morphology? Are there further successive mutations downstream in the Math-1 signaling pathway? Are there subsequent epigenetic modifications, chromatin remodeling and inactivation of tumor suppressor genes which further amplify the carcinogenesis?
Further investigations at these levels are needed that may lead to our understanding of the pathogenesis of these tumors and may have therapeutic implications. Targeted therapies to activate Notch signaling with varying concentrations for metastatic GCC may have potential benefits. The origin of goblet cells carcinoid and its transformation from typical GCC and to advanced signet ring cell, poorly differentiated adenocarcinoma could be due to spontaneous, sporadic mutation in the mentioned crypt-villus architecture and or the surrounding mesenchyme and is yet to be successfully identified. Characterizing the levels of expression of Notch pathway components in tumor samples from patients with GCC could serve as a tumor marker. This reinforces the need to further investigate the presence of these mutations in larger cohorts and in institutions treating patients with GCC and appendiceal NETs.
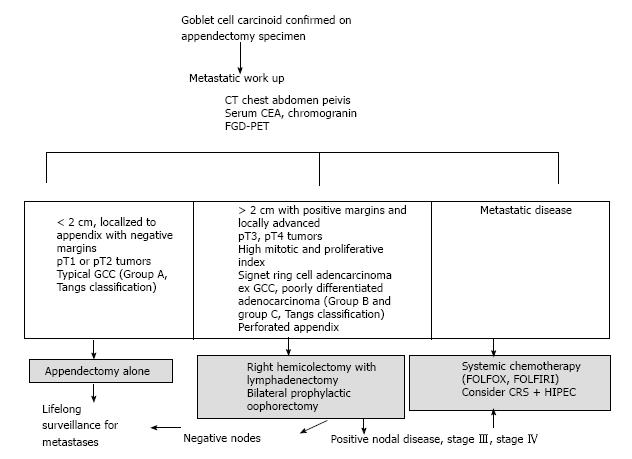
Most patients typically present with acute appendicitis and undergo appendectomy. The dilemma arises after GCC is diagnosed and confirmed, whether simple appendectomy is adequate or further oncologic resection is required (Figure 3). A multi-disciplinary evaluation is recommended for the optimal treatment. Both European and North American Neuroendocrine tumor societies guidelines recommend right hemicolectomy after appendectomy due to the high rate of metastases and its impact on prognosis[30,51]. However other authors have argued against right hemicolectomy in their series[8,13,52,53]. In a meta-analysis evaluating 13 studies with 100 patients, the authors concluded no benefits of right hemicolectomy in all patients. Selective criteria were recommended[53]. In another recent retrospective analysis of a larger number of appendiceal NETs, GCC and signet ring cell adenocarcinoma from seer database showed a benefit of right hemicolectomy and statistically improved survival only for signet ring cell cancer when compared to appendectomy alone (P = 0.01). There was no significant difference in survival for typical NETs (P = 0.21) or GCC (P = 0.94) based on type of surgery[54]. Based on Tangs classification the histology of the tumor in the appendectomy specimens and not the size of the tumor should determine the extent of oncologic resection[8]. In patients who fulfill all the following criteria: Tumor less than 2 cm localized to appendix with negative margins, pT1 or pT2 tumors, and typical GCC histology group A (Tang et al[8] classification) tumors, an appendectomy alone may be sufficient as the definitive treatment[13]. Right hemicolectomy is recommended in tumors greater than two centimeters, locally advanced, positive margins, T3, T4 tumors and histology suggestive of group B, group C (Tangs classification) in the appendectomy specimens[8,11,12,55].
The impact of perforated appendicitis in patients with GCC remains unclear. In a meta-analysis of 18 cases of GCC diagnosed upon perforated appendicitis showed no impact on survival and prognosis[56]. In another retrospective series of 20 GCC patients with perforated appendicitis, a lower rate of peritoneal metastases was observed in the perforated group (15%) compared to the non-perforated group (42%) with no difference in peritoneal relapse between the two groups[9].
A complication of GCC of the appendix is their propensity to spread to the ovaries. GCC of the appendiceal origin express elevated MUC2 and MUC5AC. In contrast mucinous tumors arising from ovarian primaries express only MUC5AC[57]. This could be of benefit in differentiating the origin of these tumors in females with primary ovarian mucinous malignancy[58]. In postmenopausal female patients with GCC prophylactic bilateral oophorectomy, although not evidence based should be considered[7,8,12]. In female patients with mucinous ovarian and pelvic malignancies an appendectomy should always be performed in staging laparotomy as these may represent metastatic GCC[12,19,57].
Adjuvant systemic chemotherapy is prescribed for stage III and stage IV diseases and disease recurrence. Due to rarity of GCC a randomized control trial cannot be accomplished. Data is available from scattered anecdotal reports and small series of GCC and therefore guidelines for choice of chemotherapy is lacking. Since metastatic GCC shows clinical and histological resemblance to colorectal adenocarcinoma and not metastatic carcinoids the choice of adjuvant therapy in GCC is similar to colorectal adenocarcinoma. 5-fluorouracil (5-FU) and leucovorin based FOLFOX (5-FU, leucovorin, oxaliplatin) and FOLFIRI (5-FU, folic acid, irinotecan) chemotherapy are standard regimens recommended[11,30].
With locally advanced or recurrent peritoneal disease, cytoreductive surgery with hyperthermic intraperitoneal mitomycin and systemic chemotherapy (CRS+ HIPEC) may improve median survival[11,12,17,59,60]. In a recent study of 45 patients with GCC and peritoneal metastases who received CRS+ HIPEC, the therapy was successfully completed in 71% of patients and 3 years, overall survival (OS) was 63.4 %[60]. Another study on 26 patients report median survival of 51 mo and an overall five-year survival of 43%[17]. However a recent retrospective study on 25 patients who received CRS plus HIPEC therapy reports no reduction in relapse rates or improvement in disease free survival in either stage I and II compared to stage III and IV[9].
The other treatment options generally available for metastatic carcinoids such as interferon, somatostatin analogues (octreotide), targeted agents such as everolimus and sunitinib and radionuclide targeted therapy is not useful for metastatic GCC due to the absence of adequate uptake on Octreoscan or Gallium 68 PET scan and no confirmed mechanistic target of rapamycin or vascular endothelial growth factor pathway dysregulation.
The overall disease specific survival for all GCC subtypes is 40%-80% depending on different series[7-9,11,12,54]. The five-year survival for localized, regional and distant metastatic disease based on Tangs classification of group A, B, C are 100%, 36% and 0% respectively. This correlates with the AJCC (TNM) staging system where reported five-year survival with stage I (100%), stage II (76%), stage III (22%), stage IV (14%), respectively.
GCC are a separate entity from carcinoids and adenocarcinoma. The pathogenesis is unclear however the tumor likely arises from pluripotent intestinal epithelial crypt base stem cells. Successive mutations likely favor them into progressing and behavior similar to poorly differentiated adenocarcinoma with minimal neuroendocrine differentiation. Metastatic lesions differ from the primary appendiceal site in terms of histology and tumor aggressiveness. A multidisciplinary approach is suggested for optimal outcomes. Surgery remains the main treatment modality. Due to its heterogeneity, this tumor should not be classified according to a single system and a combination of size of the tumor (T classification), grade and mitotic index (WHO classification) and arrangement of the goblet cells, degree of atypia and desmoplasia (Tang et al[8]’s histopathologic classification) should dictate further definitive therapy. Simple appendectomy may be sufficient in early stages while right hemicolectomy is recommended for advanced tumors. CRC with HIPEC may improve survival in a select few with metastatic peritoneal disease. These tumors have an unpredictable behavior even in early stages and local recurrence and delayed metastases may be frequently seen. Therefore lifelong surveillance is warranted.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Katada K, Sergi CM S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307-3312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | McGory ML, Maggard MA, Kang H, O’Connell JB, Ko CY. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48:2264-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Gagné F, Fortin P, Dufour V, Delage C. [Tumors of the appendix associating histologic features of carcinoid and adenocarcinoma]. Ann Anat Pathol (Paris). 1969;14:393-406. [PubMed] |
| 4. | Subbuswamy SG, Gibbs NM, Ross CF, Morson BC. Goblet cell carcinoid of the appendix. Cancer. 1974;34:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Warner TF, Seo IS. Goblet cell carcinoid of appendix: ultrastructural features and histogenetic aspects. Cancer. 1979;44:1700-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Isaacson P. Crypt cell carcinoma of the appendix (so-called adenocarcinoid tumor). Am J Surg Pathol. 1981;5:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Rossi RE, Luong TV, Caplin ME, Thirlwell C, Meyer T, Garcia-Hernandez J, Baneke A, Conte D, Toumpanakis C. Goblet cell appendiceal tumors--management dilemmas and long-term outcomes. Surg Oncol. 2015;24:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Tang LH, Shia J, Soslow RA, Dhall D, Wong WD, O’Reilly E, Qin J, Paty P, Weiser MR, Guillem J. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32:1429-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Lamarca A, Nonaka D, Lopez Escola C, Hubner RA, O’Dwyer S, Chakrabarty B, Fulford P, Valle JW. Appendiceal Goblet Cell Carcinoids: Management Considerations from a Reference Peritoneal Tumour Service Centre and ENETS Centre of Excellence. Neuroendocrinology. 2016;103:500-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Deschamps L, Couvelard A. Endocrine tumors of the appendix: a pathologic review. Arch Pathol Lab Med. 2010;134:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Taggart MW, Abraham SC, Overman MJ, Mansfield PF, Rashid A. Goblet cell carcinoid tumor, mixed goblet cell carcinoid-adenocarcinoma, and adenocarcinoma of the appendix: comparison of clinicopathologic features and prognosis. Arch Pathol Lab Med. 2015;139:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Pham TH, Wolff B, Abraham SC, Drelichman E. Surgical and chemotherapy treatment outcomes of goblet cell carcinoid: a tertiary cancer center experience. Ann Surg Oncol. 2006;13:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Bucher P, Gervaz P, Ris F, Oulhaci W, Egger JF, Morel P. Surgical treatment of appendiceal adenocarcinoid (goblet cell carcinoid). World J Surg. 2005;29:1436-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Shenoy S. Gastrointestinal carcinoids and colorectal cancers: is it a paracrine effect? Tumori. 2013;99:e141-e143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Warkel RL, Cooper PH, Helwig EB. Adenocarcinoid, a mucin-producing carcinoid tumor of the appendix: a study of 39 cases. Cancer. 1978;42:2781-2793. [PubMed] |
| 16. | Roy P, Chetty R. Goblet cell carcinoid tumors of the appendix: An overview. World J Gastrointest Oncol. 2010;2:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 17. | Yan TD, Brun EA, Sugarbaker PH. Discordant histology of primary appendiceal adenocarcinoid neoplasms with peritoneal dissemination. Ann Surg Oncol. 2008;15:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 18. | Ng D, Falck V, McConnell YJ, Mack LA, Temple WJ, Gui X. Appendiceal goblet cell carcinoid and mucinous neoplasms are closely associated tumors: lessons from their coexistence in primary tumors and concurrence in peritoneal dissemination. J Surg Oncol. 2014;109:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Toumpanakis C, Standish RA, Baishnab E, Winslet MC, Caplin ME. Goblet cell carcinoid tumors (adenocarcinoid) of the appendix. Dis Colon Rectum. 2007;50:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Olsen IH, Holt N, Langer SW, Hasselby JP, Grønbæk H, Hillingsø J, Mahmoud M, Ladekarl M, Iversen LH, Kjær A. Goblet cell carcinoids: characteristics of a Danish cohort of 83 patients. PLoS One. 2015;10:e0117627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Youn SI, Namgung H, Yun JS, Park YJ, Park DG. Peritoneal metastatic goblet-cell carcinoid tumor treated with cytoreductive surgery and intraperitoneal chemotherapy. Ann Coloproctol. 2015;31:74-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Rindi G, Arnold R, Bosman FT, Capella C, Kilmstra DS, Kloppel G, Komminoth P, Solcia E. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press 2010; 13-14. |
| 24. | Gulubova MV, Yovchev Y, Vlaykova T, Hadjipetkov P, Prangova DK, Popharitov A. Application of light microscopical and ultrastructural immunohistochemistry in the study of goblet cell carcinoid in the appendix. World J Surg Oncol. 2008;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Stancu M, Wu TT, Wallace C, Houlihan PS, Hamilton SR, Rashid A. Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol. 2003;16:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | van Eeden S, Offerhaus GJ, Hart AA, Boerrigter L, Nederlof PM, Porter E, van Velthuysen ML. Goblet cell carcinoid of the appendix: a specific type of carcinoma. Histopathology. 2007;51:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Holt N, Grønbæk H. Goblet cell carcinoids of the appendix. ScientificWorldJournal. 2013;2013:543696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Liu E, Telem DA, Warner RR, Dikman A, Divino CM. The role of Ki-67 in predicting biological behavior of goblet cell carcinoid tumor in appendix. Am J Surg. 2011;202:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Alsaad KO, Serra S, Schmitt A, Perren A, Chetty R. Cytokeratins 7 and 20 immunoexpression profile in goblet cell and classical carcinoids of appendix. Endocr Pathol. 2007;18:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, Arnold R, Denecke T, Plöckinger U, Salazar R. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95:135-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 31. | Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 32. | Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 718] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 33. | Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 552] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 34. | Koo BK, Clevers H. Stem cells marked by the R-spondin receptor LGR5. Gastroenterology. 2014;147:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1039] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141:461-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 513] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4335] [Article Influence: 240.8] [Reference Citation Analysis (0)] |
| 38. | Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Chow E, Macrae F. A review of juvenile polyposis syndrome. J Gastroenterol Hepatol. 2005;20:1634-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | van Es JH, de Geest N, van de Born M, Clevers H, Hassan BA. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun. 2010;1:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1231] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 42. | Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Cortina C, Palomo-Ponce S, Iglesias M, Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N, Gallego L, Jonkheer S. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Crabtree JS, Singleton CS, Miele L. Notch Signaling in Neuroendocrine Tumors. Front Oncol. 2016;6:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137-1142; discussion 1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW, Jin N, Collins BJ, Nelkin BD, Chen H. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350-4356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Wang H, Chen Y, Fernandez-Del Castillo C, Yilmaz O, Deshpande V. Heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors: a critical look at notch signaling pathway. Mod Pathol. 2013;26:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, Nutting C, Bushnell DL, Caplin ME, Yao JC. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 52. | Byrn JC, Wang JL, Divino CM, Nguyen SQ, Warner RR. Management of goblet cell carcinoid. J Surg Oncol. 2006;94:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Varisco B, McAlvin B, Dias J, Franga D. Adenocarcinoid of the appendix: is right hemicolectomy necessary? A meta-analysis of retrospective chart reviews. Am Surg. 2004;70:593-599. [PubMed] |
| 54. | Shaib W, Krishna K, Kim S, Goodman M, Rock J, Chen Z, Brutcher E, Staley CI, Maithel SK, Abdel-Missih S. Appendiceal Neuroendocrine, Goblet and Signet-Ring Cell Tumors: A Spectrum of Diseases with Different Patterns of Presentation and Outcome. Cancer Res Treat. 2016;48:596-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Bak M, Asschenfeldt P. Adenocarcinoid of the vermiform appendix. A clinicopathologic study of 20 cases. Dis Colon Rectum. 1988;31:605-612. [PubMed] |
| 56. | Madani A, van der Bilt JD, Consten EC, Vriens MR, Borel Rinkes IH. Perforation in appendiceal well-differentiated carcinoid and goblet cell tumors: impact on prognosis? A systematic review. Ann Surg Oncol. 2015;22:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Pahlavan PS, Kanthan R. Goblet cell carcinoid of the appendix. World J Surg Oncol. 2005;3:36. [PubMed] [DOI] [Full Text] |
| 58. | O’Connell JT, Tomlinson JS, Roberts AA, McGonigle KF, Barsky SH. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol. 2002;161:551-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Mahteme H, Sugarbaker PH. Treatment of peritoneal carcinomatosis from adenocarcinoid of appendiceal origin. Br J Surg. 2004;91:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | McConnell YJ, Mack LA, Gui X, Carr NJ, Sideris L, Temple WJ, Dubé P, Chandrakumaran K, Moran BJ, Cecil TD. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: an emerging treatment option for advanced goblet cell tumors of the appendix. Ann Surg Oncol. 2014;21:1975-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |