Published online Jan 27, 2016. doi: 10.4240/wjgs.v8.i1.5
Peer-review started: May 7, 2015
First decision: August 4, 2015
Revised: September 7, 2015
Accepted: November 24, 2015
Article in press: November 25, 2015
Published online: January 27, 2016
Processing time: 268 Days and 0.7 Hours
Laparoscopic liver resection (LLR) has been progressively developed along the past two decades. Despite initial skepticism, improved operative results made laparoscopic approach incorporated to surgical practice and operations increased in frequency and complexity. Evidence supporting LLR comes from case-series, comparative studies and meta-analysis. Despite lack of level 1 evidence, the body of literature is stronger and existing data confirms the safety, feasibility and benefits of laparoscopic approach when compared to open resection. Indications for LLR do not differ from those for open surgery. They include benign and malignant (both primary and metastatic) tumors and living donor liver harvesting. Currently, resection of lesions located on anterolateral segments and left lateral sectionectomy are performed systematically by laparoscopy in hepatobiliary specialized centers. Resection of lesions located on posterosuperior segments (1, 4a, 7, 8) and major liver resections were shown to be feasible but remain technically demanding procedures, which should be reserved to experienced surgeons. Hand-assisted and laparoscopy-assisted procedures appeared to increase the indications of minimally invasive liver surgery and are useful strategies applied to difficult and major resections. LLR proved to be safe for malignant lesions and offers some short-term advantages over open resection. Oncological results including resection margin status and long-term survival were not inferior to open resection. At present, surgical community expects high quality studies to base the already perceived better outcomes achieved by laparoscopy in major centers’ practice. Continuous surgical training, as well as new technologies should augment the application of laparoscopic liver surgery. Future applicability of new technologies such as robot assistance and image-guided surgery is still under investigation.
Core tip: Liver surgery was one of the last frontiers reached by minimally invasive surgery. Surgical technique and specialized equipment evolved to overcome technical limitations, making laparoscopic liver resections (LLR) safe and feasible. Surgeons developed skills in a stepwise approach, beginning with low complexity operations for benign diseases and reaching high-complexity surgeries for malignant cases and living donor organ harvesting. Despite a cautious slow start laparoscopic liver surgery has been incorporated to practice. On the following pages the successful history of LLR is depicted, along with an updated panel of it’s current role and expected achievements.
- Citation: Coelho FF, Kruger JAP, Fonseca GM, Araújo RLC, Jeismann VB, Perini MV, Lupinacci RM, Cecconello I, Herman P. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg 2016; 8(1): 5-26
- URL: https://www.wjgnet.com/1948-9366/full/v8/i1/5.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i1.5
In the last two decades, minimally invasive liver surgery (MILS) underwent a major evolution. Willing to explore the possibility of laparoscopic liver resection (LLR), specialized centers with solid background on hepatic and laparoscopic surgery took the first steps[1,2].
Initial development of MILS was slow, withheld by three main barriers. The first limit to overcome was technical, hence the translation of conventional techniques to the laparoscopic approach was needed. For instance, organ mobilization, manual palpation, vascular dissection, vascular control and parenchymal transection were steps universally applied to open liver resection (OLR) that had to be adapted to laparoscopy. The second barrier consisted of anticipated intraoperative hazards, such as massive bleeding and the theoretical increased risk of gas embolism secondary to pneumoperitoneum. The third step toward acceptance of LLR concerned about oncological outcomes such as adequate margins, port site seeding and long-term survival[1,3-5].
The first wedge resection was reported by Reich et al[6] (1991). Since then, improvements in surgical techniques associated with technological advances significantly expanded the complexity and safety of MILS. The first laparoscopic anatomic hepatectomy was reported in 1996 simultaneously by Azagra et al[7] and Kaneko et al[8] The first laparoscopic major hepatectomy in 1997 by Hüscher et al[9]. In 2000, Cherqui et al[1] and Descottes et al[2] published the first structured case-series with results favoring laparoscopic liver operations. Many other small single center or multicenter case series emerged on the following years, with promising good results[10-12].
By the year 2007 Koffron et al[13] published the first major series showing operative results on 300 consecutive patients. On the following year a landmark meeting produced the Louisville Statement[14], the first expert consensus conference on laparoscopic liver surgery. In 2009 a comprehensive review on published series accounted that 2804 LLR had been performed worldwide[15]. Of note, previous publications reported mainly on resected benign lesions and this review showed, for the first time, a predominance of malignant cases.
The years 2010 witnessed many reports on safety and feasibility of laparoscopic operations[16-18] including complex procedures such as major hepatectomies and graft harvesting for living donor liver transplantation (LDLT)[19,20]. Clinically significant events of carbon dioxide (CO2) embolism were extremely rare[21,22] and no port seeding or peritoneal implant could be attributed to laparoscopy, dismissing many of the initial worries on LLR[23,24].
Evidence supporting MILS comes from case-series, comparative studies and meta-analyses. Only recently, a prospective randomized study was published comparing the results of LLR with the OLR[25]. There are prospective studies in course and their results are expected to provide the best scientific evidence to the already perceived superiority of MILS[26,27]. Despite the lack of evidence level 1, existing data confirms the safety, feasibility and benefits of MILS. Also, many comparative series indicated the role of laparoscopy in disease specific settings, such as benign diseases[12,17,25,28-33], hepatocellular carcinoma (HCC)[34-49] and colorectal liver metastases (CRLM)[50-58]. Recently, the 2nd International Consensus Conference (2nd ICC) on LLR in Japan demonstrated the progress and dissemination of the method worldwide[59].
LLR should be classified according to the complexity of the operation and the technique of minimally invasive access[14]. Referring to complexity categories, laparoscopic operations might consist of: (1) small wedge resections; (2) resections of the left lateral section (segments 2 and 3) or anterior segments (segments 4b, 5 and 6); and (3) hemihepatectomies, trisectionectomies and resections of the difficult postero-superior segments (segments 1, 4a, 7 and 8). The first two categories are classified as “minor resections”. The Louisville Statement[14] defined the third group of operations as “major hepatectomies”, unlike the classical concept of open surgery, in which major resections are defined as operations resecting 3 or more contiguous liver segments[60]. Subsequently, other authors have shown that resection of lesions located in the posterior and superior segments require greater technical proficiency and have higher complication rates than anterolateral resections. These operative results justify the “upgrade” of difficult resections to major resections due to their technical complexity[61,62]. In a recent study, Di Fabio et al[62] evaluated the outcomes of the “traditional” major hepatectomies (hemi-hepatectomy, trisectionectomies) compared to laparoscopic “postero-superior” resections and concluded that the creation of two subcategories of laparoscopic major hepatectomy seems appropriate to reflect differences in intraoperative and postoperative outcomes between those two sets of patients. In the 2nd ICC the classical definition was used (minor resection: ≤ 2 segments, major resections > 2 segments)[59]. In this review, we employ the term “major resection” for hemi-hepatectomies and trisectionectomies and the term “difficult resections” for resections of the postero-superior segments (segments 1, 4a, 7 and 8).
LLR can be categorized in three different approaches[14]: (1) Pure LLR (PLLR): the entire resection is performed through laparoscopic ports and an auxiliary incision is used only for specimen extraction; (2) Hand-assisted laparoscopy surgery (HALS): The operation is carried out with elective placement of an auxiliary hand-port, which also aids specimen extraction. If a pure laparoscopic procedure demands the insertion of a hand-port in order to overcome difficulties and to complete the procedure, this should be considered a “conversion from pure laparoscopy to hand-assisted hepatectomy”. The third type of minimally invasive liver resection is (3) Hybrid hepatectomy (also termed laparoscopy-assisted hepatectomy): The operation begins with laparoscopic liver mobilization (with or without hand-assistance), followed by an elective mini-laparotomy for a conventional approach to vascular pedicles (if necessary) and parenchymal transection.
Along the development of MILS the pure laparoscopic approach was the overall preferred method, especially in European centers[63-66]. The hybrid and hand-assisted methods are adopted more liberally to perform complex resections in United States and Japan, although there is a trending shift towards PLLR in Japan[67-70].
To overcome the difficulties associated with minimally invasive hepatic resections the training of surgeons is essential in order to safely spread the benefits of laparoscopic liver surgery[71-73].
A consensual observation in many papers on LLR is that laparoscopic hepatectomies should be performed by surgeons with extensive training in hepatobiliary and advanced laparoscopic surgery[14,74]. Thence, fellowships in specialized centers should offer high-level training in order to accomplish competence in both domains. The key points related to the training with LLR are summarized in Table 1.
| Knowledge of caudal view anatomy |
| Training in cadaveric and/or animal model |
| Clinical training should follow an increasing order of procedure difficulty (Difficult Scoring System for LLR[78] can be used to point difficulty levels) |
| Minor anterolateral resections |
| Minor anatomical resections (start with LLS) |
| Difficult resections |
| Major resections |
| Graft harvesting for LDLT |
| HALS and hybrid resections can be used in the early experience to overcome the learning curve |
A major change in MILS is the way surgeons approach the liver, as the classic open frontal view is modified. In laparoscopy, due to the insertion of the optical equipment in or near the umbilicus, a caudal approach is forcefully undertaken. In the caudal view the surgeon sees the well-known anatomy from a different perspective[64]. Basic technical skills acquisition can occur through practicing in cadaveric or animal model[75] and further clinical training should follow an increasing order of procedure difficulty[14,73,76]. Case selection is essential in early clinical experience; first cases should involve lesions prone to small wedge resections located on the anterolateral segments (segments 2, 3, 4b, 5 and 6). Anatomic resections can be started with left lateral sectionectomy (LLS), which is a patterned straightforward segmental resection that requires liver mobilization, pedicle treatment and parenchymal transection[33,71,76,77]. It is safer to move on to complex resections after the surgeon has acquired proficiency in minor resections (Table 1)[73].
In order to better understand the difficulty associated with each kind of operation, a recent paper proposed a scoring index for LLR[78]. This scoring system incorporates factors such as tumor size and location; proximity to major vessels; liver function and extent of liver resection. Resections are graded from 1 to 10, being score 1 for peripheral wedge resection; 4 for LLS; 7 for hemihepatectomy and 10 stands for extremely difficult resections. This interesting index offers a numeric score of progressive difficulty that can help learners to evaluate their progress. This scoring system can be used as a guide in training and progressive skill acquisition.
Learning curve analysis is somewhat a preoccupation linked to laparoscopic operations. When a laparoscopic operation is proposed, it is usually compared with its conventional counterpart in order to assess results and to establish the number of operations required for technical competence. To our knowledge there is no data on open hepatectomy learning curve, and Rau et al[79] published the first mention of a LLR learning curve in 1998. Cherqui’s group published a seminal paper on the subject and their analysis revealed that 60 cases were needed in order to achieve optimal results[72]. Of notice, the use and duration of Pringle maneuver, and use of HALS decreased over time. This indicates that pedicle clamping and HALS play an important role during the learning curve. Likewise, hybrid resections can also be used in the early experience to overcome the learning curve[13,70,80].
Other series have indicated a smaller number in order to obtain expertise. A recent Chinese paper observed a variable number on cases needed to achieve competence, according to the complexity of the operation. Their caseload ranged between 15 to 43 operations[81].
Other authors have made some interesting conjoined analysis, looking beyond the numbers, also considering the effect of expert training. Hasegawa et al[58] made an analysis on their experience with 24 cases of LLS divided in 3 eras; initially a senior surgeon performed the first 8 operations with no technical standardization. In the second era a senior surgeon operated on 8 cases with a standardized technique. The third group of 8 patients underwent operations performed by junior surgeons under senior guidance. Comparative analysis showed better results of the second and third eras in comparison to the first period and, most important, results did not differ between the second and third periods.
Other authors studied the learning curve for complex and major hepatic resections[73,82]. Nomi et al[83] studied 173 patients that underwent major LLR in a high-volume center using the cumulative sum method. The learning curve identified three phases: Phase 1 (45 initial patients), phase 2 (30 intermediate patients) and phase 3 (the subsequent 98 patients). These data suggests that the learning phase of major LLR included 45 to 75 patients[83].
Laparoscopic access offers some benefits over conventional operations[66,84]. The magnified view of the operating field allows meticulous haemostasis. During parenchymal transection, most of the blood loss derives from the hepatic veins and laparoscopy offers the possibility of parenchymal transection under positive pressure, resulting in less bleeding. However, minimally invasive liver operations has some drawbacks, such as lack of tactile feedback, restricted manoeuvres and difficult visualization of the posterior and superior segments of the liver[85].
Nowadays, LLR is utilized in a small percentage of liver resections (5%-30%) in most centers, although some very skillful surgeons have reported higher rates, reaching from 50% to 80%[59,64,86]. The majority of data arise from minor resections but the proportion of major resections is increasing[64]. A recent analysis performed in a general medical population, including all liver resections in France along the year 2013, resulted that 15% of liver resections were performed through minimally invasive surgery[87]. Another surgical population profile indicates that less than 10% of all liver resections done for benign conditions are carried out with laparoscopy in North American centers[88]. In fact, there are few centers with extensive experience with LLR, Table 2 presents per operative results in high-volume centers with more than 150 cases.
| Ref. | Cases | Malignancy | Minimally invasive approach (%) | Major resections | Conversion | Operative time | Blood loss | Transfusion | LOS | Complication | Mortality | ||
| (n) | (%) | PLLR | HALS | Hybrid | (%) | (%) | (median/mean, min) | (median/mean, mL) | (%) | (median/mean, d) | (%) | (%) | |
| Koffron et al[13] | 300 | 34 | 80.3 | 10.6 | 9 | 39 | 7.3 | 99 | 102 | 0.6 | 1.9 | 9.3 | 0 |
| Buell et al[165] | 306 | 42 | NR | NR | NR | 45 | 2.4 | 162 | 222 | 7 | 2.9 | 16 | 1.6 |
| Bryant et al[163] | 166 | 60 | 95.2 | 4.8 | 0 | 18.6 | 9.6 | 180 | 329 | 5.4 | 6 | 15.1 | 0 |
| Long et al[164] | 173 | 100 (HCC) | 100 | 0 | 0 | 8.3 | 2.3 | 112 | 194 | NR | 6.5 | 2.4 | 0 |
| Cai et al[81] | 365 | 27.1 | 100 | 0 | 0 | 22.2 | 17.2 | 150 | 370 | NR | 9.2 | 12.2 | 0 |
| Gobardhan et al[93] | 476 | 79 | NR | NR | NR | 33.8 | 4.2 | NA | NA | 4.6 | NR | 21 | 0.8 |
| Troisi et al[99] | 265 | 64.1 | 99.3 | 0 | 0.7 | 17.4 | 6.4 | 254 | 171 | NR | 5.5 | 14 | 0 |
| University of Sao Paulo series 2015 | 214 | 65.9 | 75.2 | 5.6 | 19.2 | 14.5 | 6.5 | 205 | 240 | 7.4 | 4.5 | 15 | 0.5 |
Indications for LLR do not differ from those for open surgery[3,89,90]. Indications are based on tumour characteristics, liver function and patient’s general health status. In patients who cannot tolerate pneumoperitoneum due to their cardiopulmonary status LLR is contraindicated.
MILS may be used for benign and malignant (primary and metastatic) tumors and living donor liver harvesting. A high percentage of benign tumors was presented in early series of MILS, whereas the proportion of malignant has significantly increased in recent years[15,79,91,92]. Between June 2007 and December 2014, 214 LLR were performed at the Liver Surgery Unit, University of Sao Paulo Medical School. In our experience, 65.9% of LLR patients were by malignant diseases and their proportion has significantly increased in recent years (Table 2).
Classic indications for LLR are tumours confined to the so-called “laparoscopic segments”: The left lateral section (segments 2 and 3) and the anterior segments (segments 4b, 5, 6). It is also preferable to operate on tumours smaller than 5 cm, located away from major blood vessels and hilar structures. Those are the most frequently adopted indications, but are not restrictive, once indications can be shifted and extent of resection can be expanded according to the expertise of a particular center[85,93]. For instance, peripheral tumours are amenable to laparoscopic approach, even when greater than 5 cm. On the other hand, LLR is not advised for large intrahepatic lesions, because of difficult tumor mobilization and risk of rupture[3]. Laparoscopic LLS is considered the gold standard treatment for lesions located on segments 2 and 3 and should be routinely applied[33,71,94]. This successful policy has led some experts to expand the indication of routine laparoscopic approach to left hepatectomy[95].
Major liver resections (i.e., right hepatectomy) showed to be feasible but remain technically demanding procedures reserved to experienced surgeons. Patients with bilateral or central tumors, close to the liver hilum, major hepatic veins or inferior vena cava (IVC) are not standard candidates for a laparoscopic approach. However, in some expert centers, even these cases are addressed laparoscopically in selected patients[96,97].
Posterior and superior segments of the liver have been traditionally considered as “non-laparoscopic segments” because laparoscopy offers a caudal vision of the liver and there is a great amount of parenchyma interposed between the surgeon’s view and those segments[84,98]. The combination of oddly located lesions and extensive liver mobilization implies in worst operative outcomes, such as prolonged operative time, higher blood loss and narrower margins[61,99,100]. Moreover, lesions located on the posterior and superior segments have been identified as an independent risk factor for bleeding and conversion[99].
Fortunately, there are strategies to overcome limitations of laparoscopy, as some authors have pointed out, HALS and hybrid procedures are safe methods of performing “difficult” and major resections[68,69]. Other alternative accesses include the superior and lateral approaches with or without use of intercostal or transthoracic trocars. These techniques offer direct access to superior segments, but they have been performed only in few expert centres with small case series reported[101-104].
Benign diseases: There is general consensus that indications for benign lesions should not be expanded in face of lesser invasive technology[14]. Symptomatic benign diseases (complex cystic diseases, haemangioma, focal nodular hyperplasia) or risk bearing tumours (hepatocellular adenomas) might be suitable for LLR. Cases of segmental hepatolithiasis associated with parenchymal atrophy are also good candidates for LLR[105]. Some series have demonstrated the feasibility and safety of LLR for benign diseases with low morbidity (< 20%) and no mortality (Table 3)[12,17,25,28-33]. There are few comparative studies between LLR and OLR for benign tumours[30,33], showing significantly reduced blood loss, time to oral intake, hospital stay and total cost of hospitalization in patients that underwent LLR. Recently, Ding et al[25] published a randomized trial comparing patients with hepatolithiasis undergoing laparoscopic and OLR, showing benefits for the LLR group. In the authors’ experience with 73 LLR for benign liver diseases, the main indication was hepatocellular adenoma (HA) (n = 50; 68.5%). There were 11 (15.1%) major resections: 9 right and 2 left hepatectomies. Blood transfusion was required in 4.1% of patients and there was no need for conversion. Postoperative complications occurred in 6.9% of the patients and operative mortality was nil (Table 3).
| Ref. | Cases (n) | Type of study | Major hepatectomies (%) | Conversion (%) | Transfusion (%) | Complication (%) | Mortality (%) |
| Katkhouda et al[28] | 121 | Retrospective | 0 | 8.3 | 0 | 0 | 0 |
| Descottes et al[12] | 87 | Retrospective multicenter | 3.4 | 10 | 6 | 5 | 0 |
| Ardito et al[29] | 50 | Retrospective | 16 | 8 | 2 | 10 | 0 |
| Troisi et al[30] | 20 | Comparative (LLR × OLR) | 2.5 | 10 | 10 | 20 | 0 |
| Abu Hilal et al[32] | 50 | Retrospective | 28 | 2 | 2 | 7 | 0 |
| Abu Hilal et al[31] | 13 HA only | Retrospective | 62 | 0 | 7.7 | 7.7 | 0 |
| Herman et al[17] | 31 HA only | Retrospective | 16.7 | 0 | 0 | 6.5 | 0 |
| de'Angelis et al[197] | 36 HA only | Comparative (LLR × OLR) | 25 | 8.3 | 0 | 8.3 | 0 |
| Dokmak et al[33] | 31 LLS only | Comparative LLS (LLR × OLR) | 0 | 6.5 | NR | 9.7 | 0 |
| Ding et al[25] | 49 hepatolithiasis only underwent LLS | Prospective randomized trial (LLR × OLR) | 0 | 4.1 | NR | 6.1 | 0 |
| University of Sao Paulo series 2015 | 73 | 15.1 | 0 | 4.1 | 6.9 | 0 |
Despite rare benign tumours, HA are clinically relevant due to the risk of malignant degeneration and bleeding. HA have specific indications for surgery such as male sex and lesion larger than 5 cm or symptomatic in females[17,106]. When operation is required, LLR has proved to be safe and feasible, even when major resections were required[17,31]. In a recent series by our group, we found excellent results using PLLR, with low rate of operative complications, without mortality or long-term recurrence[17].
This group of patients seems to be specially benefited by laparoscopic surgery, once this disease occurs in patients along their productive life where a less invasive approach offers faster recovery, shorter hospital stay, lower morbidity and better cosmetic result. LLR should be considered standard of care for patients with adenomas, when performed by experienced laparoscopic liver surgeons[17].
HCC usually occurs in the setting of chronic liver disease and liver transplantation (LT) would offer adequate treatment for HCC as well as for the underlying disease. In patients with preserved liver function or limited signs of portal hypertension, resection is the mainstay treatment and provides the same results in an intention-to-treat fashion that LT offers[66,107].
In the early experience with LLR the chronically diseased liver was believed to increase the feared hazard of intraoperative bleeding[108,109]. However, some initial series demonstrated the safety and feasibility of LLR in selected patients[34-46,48,49,110]. Pioneer specialized centers performed minimally invasive resections on patients with preserved liver function (Child-Pugh class A), limited signs portal hypertension (platelet count over 80000-100000; oesophageal varices grade 1 or less; absence of ascites) and good general health (ASA III or less)[65,66].
In addition to liver function, tumour characteristics also restrict the use of laparoscopy in the treatment of HCC. In Tranchart series, case selection resulted in 27% of HCC patients treated with minimally invasive approach[35]. Usually, centrally located lesions are managed via ablative techniques and larger resections (> 2 segments) are rarely carried out. Laparoscopy is preferred for anterolateral segments, smaller tumours (less than 5 cm) and lesions far from vascular structures[111]. Anatomical resection is preferred whenever possible for the treatment of HCC in order to achieve adequate margins and prevent local recurrence. However, in small peripheral and well-differentiated HCC there are studies showing similar results for anatomic and non-anatomic resections[112-114].
Judicious patient selection improved surgical outcomes and the accumulated experience once again ruled out the initial fears of LLR for HCC[34-46,48,49,110]. Since 2000, over 600 cases of LLR of HCC were reported[23] and, interestingly, LLR results in less blood loss and less requirement for blood transfusion[86,115]. The reduced blood loss must be considered an outstanding outcome, once operative blood loss has a strong association with prognosis in HCC patients[116].
Laparoscopy also reduces operative morbidity, considering general and liver specific complications. Patients suffer fewer pulmonary complications and, importantly, there is an impressive reduction in postoperative ascites and liver insufficiency[23,109,117,118]. Laparoscopy avoids abdominal wall disruption; consequently avoiding discontinuation of the compensatory collateral circulation secondary to portal hypertension. Laparoscopic surgery also implies in limited liver manipulation, restricted fluid management, decreased blood loss and consequently reduced third space accumulation and hyper aldosteronism[111]. Table 4 summarizes operative results of MILS in comparatives series with more than 30 patients in each studied group (OLR × LLR).
| Ref. | No. of patients | Blood loss (mean/median, mL) | Blood transfusion (%) | Conversion (%) | Complication (%) | Perioperative mortality (%) | 5-yr overall survival (%) | 5-yr recurrence free survival (%) | |||||||||||||
| LLR | OLR | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | ||
| Belli et al[34] | 54 | 125 | 297 | 580 | < 0.01 | 11 | 25.6 | 0.03 | 7 | 19 | 36 | 0.02 | 2 | 4 | NS | 671 | - | NS | 521 | - | NS |
| Tranchart et al[35] | 42 | 42 | 364 | 723 | < 0.0001 | 9.5 | 16.7 | NS | 4.7 | 21.4 | 40.5 | NS | 2.4 | 2.4 | NS | 59.51 | 47.41 | NS | 60.91 | 54.31 | NS |
| Truant et al[36] | 36 | 61 | 452 | 447 | NS | 2.8 | 3.8 | NS | 19.4 | 25 | 35.8 | NS | 0 | 7.5 | NS | 70 | 46 | NS | 35.5 | 33.6 | NS |
| Lee et al[37] | 33 | 50 | 150 | 240 | NS | 6.1 | 10 | NS | 18.2 | 6.1 | 24 | 0.033 | 0 | 0 | NS | 76 | 76.1 | NS | 45.3 | 55.9 | NS |
| Hu et al[110] | 30 | 30 | 520 g | 480 g | NS | NA | NA | - | 0 | 13.3 | 10 | NS | 0 | 0 | NS | 50 | 50.3 | NS | NR | NR | - |
| Ker et al[38] | 116 | 208 | 139 | 1147 | < 0.001 | 6.9 | 50.9 | < 0.001 | 5.2 | 6 | 30.2 | < 0.001 | 0 | 2.9 | NS | 62.2 | 71.8 | NS | NR | NR | - |
| Cheung et al[39] | 32 | 64 | 150 | 300 | 0.001 | 0 | 4.7 | NS | NR | 6.3 | 18.8 | NS | 0 | 1.6 | NS | 76.6 | 57 | NS | 54.5 | 44.3 | NS |
| Ai et al[40] | 97 | 178 | 460 | 454 | NS | 4.6 | 2.8 | NS | 9.28 | 9 | 30 | 0.001 | 0 | 0 | NS | 861 | 881 | NS | 661 | 671 | NS |
| Memeo et al[41] | 45 | 45 | 200 | 200 | NS | 0 | 0 | NS | NR | 20 | 45 | 0.01 | 2 | 13 | 0.04 | 59 | 44 | NS | 19 | 23 | NS |
| Kim et al[42] | 70 | 70 | NA | NA | - | 24.3 | 40.8 | 0.001 | 8.57 | 7.1 | 14.5 | NS | NR | NR | - | 65.3 | 65.7 | NS | 58.3 | 62.6 | NS |
| Kim et al[198] | 43 | 162 | 484 | 261 | NS | 3.4 | 0 | NS | 23.3 | 13.8 | 37.9 | NS | 0 | 0 | NS | 92.2 | 87.7 | NS | 54 | 40.1 | NS |
| Ahn et al[43] | 51 | 51 | 350 | 355.2 | NS | 5.9 | 9.8 | NS | Excluded | 5.8 | 9.8 | NR | 0 | 0 | NS | 80.1 | 85.7 | NS | 67.8 | 54.8 | NS |
| Yamashita et al[44] | 63 | 99 | 455 | 436 | NS | 6 | 2 | NS | NA | 10 | 26 | 0.046 | 0 | 0 | NS | 69 | 77 | NS | 33 | 41 | NS |
| Yoon et al[45] | 58 | 174 | NA | NA | - | 3.4 | 7.5 | 0.04 | 0 | 6.9 | 22.4 | 0.02 | NR | NR | - | 882 | 682 | NS | 562 | 622 | NS |
| Martin et al[46] | 100 | 254 | 336 | 755 | < 0.001 | 1.2 | 2.4 | 0.043 | NR | 44 | 57 | NS | 6 | 8 | NS | 60.71 | 41.81 | NS | 201 | 261 | NS |
| Lee et al[47] | 43 | 86 | 300 | 700 | 0.004 | NR | NR | - | 14 | 23.3 | 39.5 | NS | NR | NR | - | 89.7 | 87.3 | NS | 53.5 | 58.6 | NS |
| Han et al[48] | 88 | 88 | 500 | 525 | NS | 20 | 26.1 | NS | 9.1 | 12.5 | 20.4 | 0.042 | 1.1 | 1.1 | NS | 76.4 | 73.2 | NS | 44.2 | 41.2 | NS |
| Xiao et al[49] | 41 | 86 | 272.2 | 170.8 | 0.001 | 7.3 | 13.9 | NS | 7.32 | 17.1 | 37.3 | 0.021 | 0 | 0 | NS | 781 | 76.71 | NS | 70.71 | 68.61 | NS |
Another beneficial implication of LLR in cirrhotic patients is better results of LT after laparoscopic resection when compared with OLRs[119]. After laparoscopic procedures there are fewer adhesions, which ultimately translates as a shorter dissection time, with less bleeding from hypervascularized adhesions associated to portal hypertension. Therefore LLR resulted in shorter operative time, less blood loss and quicker hepatectomy phase during LT[119,120]. Other studies also pointed out that reoperations for recurrent HCCs are feasible and facilitated by a previous LLR[121-123]. This is a clear advantage in the setting of chronic liver disease, where patients are prone to develop new tumours or hepatic insufficiency, requiring further resection or LT.
Resection is considered the gold standard treatment for CRLM and offers the best chance of long-term survival[124,125]. Many of the oncological fears were shared with HCC and included surgical margins, adequate intraoperative staging, peritoneal dissemination and port site seeding.
Laparoscopic treatment of CRLM was one of the most recent achievements of MILS. Until 2008, only 35% of LLR for malignancy were performed for CRLM[14]. The first multi-institutional cohort with 109 patients was published only in 2009[126], with a stringent selection criteria: tumours smaller than 5 cm, lesions located on the peripheral segments and multiple lesions were treated only if tumor clearance could be achieved with a single anatomic resection. The transfusion rate was 10%, the resection margin was negative in 94.4% of the patients and the conversion rate was 3.7%[126].
Case series and comparative studies indicated that well selected patients presented reduced morbidity and blood loss[50-58] (Table 5). Recent meta-analysis confirmed the benefits of laparoscopy approach when compared to OLR (Table 6)[127-130].
| Ref. | No. of patients | Blood loss (mean/median, mL) | Blood transfusion (%) | Conversion (%) | Complication (% ) | Perioperative mortality (%) | 5-yr overall survival (%) | 5-yr recurrence free survival (%) | |||||||||||||
| LLR | OLR | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | LLR | OLR | P value | ||
| Castaing et al[50] | 60 | 60 | NR | NR | - | 15 | 36 | 0.007 | 10 | 27 | 28 | NS | 1.7 | 1.7 | NS | 64 | 56 | NS | 30 | 20 | NS |
| Abu Hilal et al[51] | 55 | 119 | 363 | 500 | NR | 3.6 | NR | NR | 12 | 15 | 20.2 | NR | 0 | 2 | NR | NR | NR | NR | NR | NR | - |
| Cannon et al[52] | 35 | 140 | 202 | 392 | < 0.001 | 17 | 25 | NS | NR | 23 | 50 | 0.004 | 0 | 1.4 | NS | 36 | 42 | NS | 15 | 22 | NS |
| Qiu et al[53] | 30 | 140 | 215 | 390 | < 0.001 | 10 | 25 | < 0.05 | 6.6 | 26.2 | 55 | < 0.001 | 0 | 0 | NS | NR | NR | - | NR | NR | - |
| Guerron et al[54] | 40 | 40 | 376 | 753 | 0.041 | 5 | 20 | 0.04 | 5 | 15 | 20 | NS | 0 | 0 | NS | NR | NR | NS | NR | NR | NS |
| Kubota et al[55] | 43 | 62 | 287.3 | 579.3 | < 0.001 | 2.4 | 21 | 0.004 | NR | 2.4 | 9.7 | NS | 0 | 0 | NS | 88.41 | 74.21 | NS1 | NR | NR | - |
| Montalti et al[56] | 57 | 57 | 454.2 | 691.5 | 0.003 | NR | NR | - | 15.8 | 15.8 | 31.6 | 0.03 | 0 | 0 | NS | 60 | 65 | NS | 29 | 38 | NS |
| de'Angelis et al[57] | 52 | 52 | 200 | 300 | 0.001 | 5.8 | 21.2 | NS | 5.8 | 17.3 | 22 | NS | 0 | 3.8 | NS | 73.1 | 62.5 | NS | 21.1 | 21.1 | NS |
| Hasegawa et al[58] | 102 | 69 | 127 | 620 | 0.000 | NR | NR | NR | 1 | 8.8 | 24.6 | 0.005 | 0.98 | 1.4 | NS | 56.8 | 48.8 | NS | 39.7 | 28.6 | NS |
| Ref. | No. of studies/period | Type of studies | End point | No. of OLR × LLR | Conversion rate | Favors ORL | Favors LLR | Equal OLR × LLR |
| Simillis et al[151] | 8 | Observational | Short-term outcomes | 244 (59.7%) × 165 (40.3%) | 3.7% | Need and duration of portal clamping | Operative blood loss | Blood transfusion rate |
| Nonrandomized | Return to oral intake | Operative time | ||||||
| Comparative | LOS | Incidence of portal triad clamping | ||||||
| 1998-2005 | Benign and malignant disease | Adverse events | ||||||
| Oncologic clearance | ||||||||
| Croome et al[150] | 26 | Observational | Short and long-term outcomes | 1019 (53.9%) × 871 (46.1%) | 3.5% | Lower risk of margins smaller than 1 cm | Operative blood loss | Blood transfusion rate |
| Nonrandomized | Return to oral intake | Operative time | ||||||
| Comparative | Need for intravenous narcotics | Incidence of portal triad clamping | ||||||
| 1998-2009 | Benign and malignant disease | Overall complication rate | Oncologic clearance | |||||
| All cause mortality in 2-5 yr | Recurrence risk | |||||||
| Mirnezami et al[149] | 26 | Observational | Short and long-term outcomes | 961 (57%) × 717 (43%) | 7% | Operative time | Operative blood loss | Portal trial clamping time |
| Nonrandomized | Return to oral intake | Margin size | ||||||
| Comparative | Overall and specific complications | Hepatic tumor recurrence for HCC | ||||||
| 1998-2009 | Benign and malignant disease | Incidence of portal triad clamping | DFS for HCC | |||||
| Lower risk of margins smaller than 1 cm | ||||||||
| Lower rate of positive margins | ||||||||
| Increased overall survival for HCC | ||||||||
| Zhou et al[118] | 10 | Observational | Short and long-term outcomes | 281 (56.9%) × 213 (43.1%) | 6.1% | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | Postoperative mortality | ||||||
| Comparative | Overall and liver specific complications | Size of resection margins | ||||||
| 2001-2010 | HCC only | LOS | DFS | |||||
| Overall survival | ||||||||
| Mizuguchi et al[152] | 11 | Observational | Short-term outcomes | 253 (52.2%) × 232 (47.8%) | NR | - | Operative blood loss | Operative time |
| Nonrandomized | Postoperative complications | |||||||
| Comparative | LOS | |||||||
| 2001-2008 | Benign and malignant disease | |||||||
| Fancellu et al[111] | 9 | Observational | Short and long-term outcomes | 363 (61.5%) × 227 (38.5%) | 4% | - | Rate of positive margins | Liver haemorrhage |
| Nonrandomized | Operative blood loss | Bile leakage | ||||||
| Comparative | Blood transfusion rate | OS | ||||||
| 2001-2010 | HCC only | LOS | DFS | |||||
| Overall and liver specific complications | ||||||||
| Rao et al[94] | 7 | Observational | Short-term outcomes | 111 (45.3%) × 134 (54.7%) | 2.7% | - | Postoperative complications | Operative blood loss |
| Nonrandomized | Operative time | Blood transfusion rate | ||||||
| 2005-2009 | Comparative only patients undergoing laparoscopic × open LLS | LOS | Rate of positive margins | |||||
| Li et al[173] | 10 | Observational | Short and long-term outcomes | 383 (61%) × 244 (39%) | 6.6% | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | Surgical margin size | ||||||
| Comparative | Postoperative complications | Rate of positive margins | ||||||
| 2001-2011 | HCC only | LOS | Tumor recurrence | |||||
| Rao et al[148] | 32 | Observational | Short and long-term outcomes | 1305 (52.9%) x 1161 (47.1%) | 2.3% | Operative time | Operative blood loss | Overall and liver specific complications |
| Nonrandomized | Blood transfusion rate | Operative mortality | ||||||
| Comparative | LOS | Margin width | ||||||
| 1998-2009 | Benign and malignant disease | Return to oral intake | DFS | |||||
| Rate of positive margins | OS | |||||||
| Overall complication rate | Recurrence rate | |||||||
| Xiong et al[117] | 15 | Observational | Short and long-term outcomes | 316 (57.5%) × 234 (42.5%) | 0% to 19.4% | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | Pulmonary complications | ||||||
| Comparative | LOS | Bleeding | ||||||
| 2001-2011 | HCC only | Incidence of liver failure and ascites | Bile leakage | |||||
| Operative mortality | ||||||||
| Rate of positive margins | ||||||||
| Tumor recurrence | ||||||||
| Rao et al[172] | 10 | Observational | Short-term outcomes | 404 (57.7%) × 296 (42.3%) | NR | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | Ascitis | ||||||
| 2002-2009 | Comparative | Negative margins rate | Postoperative liver failure | |||||
| Malignant tumors | Overall complication rate | DFS | ||||||
| LOS | OS | |||||||
| Recurrence rate | ||||||||
| Yin et al[174] | 15 | Observational | Short and long-term outcomes | 753 (60.8%) × 485 (39.2%) | 2.6% | - | Operative blood loss | Surgical margins |
| Nonrandomized | Blood transfusion rate | Operative time | ||||||
| Comparative | Postoperative morbidity | Negative margins rate | ||||||
| 2001-2011 | HCC only | LOS | Recurrence rate | |||||
| OS | ||||||||
| RFS | ||||||||
| Zhou et al[129] | 8 | Observational | Short and long-term outcomes | 427 (61.4%) × 268 (38.6%) | 8.2% | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | |||||||
| Comparative | Postoperative morbidity | |||||||
| 2002-2013 | CRLM only | LOS | ||||||
| Negative margin | ||||||||
| OS | ||||||||
| DFS | ||||||||
| Twaij et al[168] | 4 | Observational | Short and long-term outcomes | 270 (64.3%) × 150 (35.7%) | 7% to 19.4% | - | Surgical margin size | Operative time |
| Nonrandomized | Operative blood loss | OS | ||||||
| Comparative | Blood transfusion rate | DFS | ||||||
| 2009-2013 | HCC only with cirrhosis | Postoperative morbidity | ||||||
| Parks et al[167] | 15 | Observational | Short and long-term outcomes | 556 (55.5%) × 446 (44.5%) | 4.2% | - | Operative blood loss | Operative time |
| Nonrandomized | LOS | 30-d mortality | ||||||
| Comparative | OS | |||||||
| 2001-2011 | Malignant tumors | |||||||
| Luo et al[128] | 7 | Observational | Short and long-term outcomes | 383 (61.4%) × 241 (38.6%) | NR | Margin size | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | LOS | ||||||
| Comparative | Postoperative complications | OS | ||||||
| 2002-2013 | CRLM only | Rate of R1 margins | DFS | |||||
| Wei et al[127] | 14 | Observational | Short and long-term outcomes | 599 (61.4%) × 376 (38.6%) | NR | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | Perioperative mortality | ||||||
| 2002-2013 | Comparative | LOS | OS | |||||
| CRLM only | Postoperative complications | DFS | ||||||
| 13 studies comparing simultaneous | ||||||||
| laparoscopic × open resections | 1Simultaneous resection | 1Simultaneous resection | ||||||
| LOS | Morbidity, operative time, operative blood loss | |||||||
| Schiffman et al[130] | 8 | Observational | Short and long-term outcomes | 368 (60.3%) × 242 (39.7%) | NR | - | Operative blood loss | Operative time |
| Nonrandomized | Blood transfusion rate | Number of major resections | ||||||
| Comparative | Overall complication rate | Mean number of resected tumors | ||||||
| 2009-2013 | CRLM only | LOS | Tumor size | |||||
| Positive margins | ||||||||
| Margin width | ||||||||
| OS | ||||||||
| DFS | ||||||||
| Jackson et al[153] | 46 | Observational | Short-term outcomes | 1741 (47.8%) × 1901 (52.2%) | 5.68% | Cost | Operative blood loss | Operative time |
| Nonrandomized | Negative margin | 30-d mortality | ||||||
| 2001-2013 | Comparative | LOS | ||||||
| Benign and malignant disease | Postoperative complications | |||||||
| Morise et al[169] | 21 | Observational | Ascitis | 602 (63.1%) | NR | - | Less ascites | - |
| Nonrandomized | × 352 (36.9%) | Less postoperative liver failure | ||||||
| 2001-2014 | Comparative | |||||||
| HCC only | Postoperative liver failure | 220 (54.6%) × 183 (45.7%) |
A recent paper made an interesting matched pair analysis of open and laparoscopic approaches for CRLM[56] and showed increased rate of third liver resections on the laparoscopy group. This confirms, once again, that laparoscopy eases further interventions, as mentioned for HCC.
Good outcomes have encouraged some specialized centres to widen the indications of MILS, for instance, in elderly patients[131]. Another progress appears related to synchronous resections of the colorectal primary tumour and CRLM. The association of these two operations appears to be feasible and safe in specialized centers[127,132,133].
Other liver malignancies such as peripheral cholangiocarcinoma, hilar cholangiocarcinoma, as well as non-CRLM have had only anecdotal reports[16,134,135]. Although considered a contraindication for LLR by most specialized centers, successful minimally invasive approach to gallbladder cancer has been performed in few cases with no reports of port site metastasis[136]. Despite being an interesting topic, to date the available data is very limited and no major conclusion can be disclosed.
Laparoscopic graft harvesting has raised the same questions on technical issues and operative hazards as other LLR. One specific limitation associated with laparoscopic organ harvesting is the risk of prolonged warm ischemia time[19], although this has not shown to be harmful and had no negative effect on graft function[65]. Thenappan et al[137] compared laparoscopic and open donor hepatectomies and found no significant differences except a minor increase in international normalised ratio on day 7 in the laparoscopic group. Perioperative allograft biliary and vascular complication rates were also comparable between groups. One-year graft and patient survival for laparoscopic group was 100% compared with 93% for the open group[137].
Cherqui et al[138] reported the first pure laparoscopic LLS for donor hepatectomy in 2002. Subsequently, studies have identified benefits for the donor as decreased blood loss, decreased postoperative complication rates and shorter postoperative recovery and hospital stay[20,65,139]. Experienced centers point out that laparoscopic LLS should be shortly accepted as standard of care[19,20].
Laparoscopic major hepatectomies are usually performed in the setting of adult-to-adult LDLT. Left liver harvesting offers a lower risk strategy for the donor while gives to the recipient a relative large amount of functioning liver parenchyma[140]. Smaller grafts tend to shift the risks from the donor to the recipient, as they increase the risk of a small for size syndrome. Technical improvements have led to better results and small series have shown that this strategy can be undertaken with low morbidity[141,142].
Right hemilivers offer great amount of functional liver parenchyma, but implies in a larger resection associated with higher risks to donors[143]. Some centers advocated that laparoscopic harvesting could decrease postoperative complication rates; however there are concerns about the safety of the procedure. Some authors advocate HALS and hybrid procedures in order to reduce donor morbidity[70,144-146]. Cauchy et al[19] reported 167 right livers harvested through laparoscopy being 98.2% operated with HALS or hybrid techniques. Results showed no mortality, and low rate of severe complications (0% to 17%).
In a recent meta-analysis by Berardi et al[147] 11 studies were included (608 adult patients) comparing minimally invasive and open living donor hepatectomy. Blood loss and operative time were comparable between groups, while hospital stay, analgesia use, donor morbidity rate and wound-related complications were significantly reduced in laparoscopic group[147].
At present, laparoscopic major hepatectomy for LDLT graft harvesting is a debatable procedure, restricted to few centers and needs further scientific confirmation. According to the 2nd ICC laparoscopic donor harvesting requires both ongoing institutional ethical approval and a reporting registry of all cases to determine the short and long-term outcomes in donors and recipients. Considering the high level of surgical skills required, it is advised to perform this procedure only at centers with experience in MILS and LT[59].
Operative time varies significantly between studies, influenced by the type of resection and surgeon’s experience. Vigano et al[72] studied three consecutive periods, each with 58 patients undergoing LLR, and observed a significant decrease in mean operative time. Other authors have confirmed the trend of significant reduction in operative time related to increasing experience, both in minor and major resections[77,82].
When compared to open approach, results are conflicting. Some studies have shown longer operative time in LLR group, including 2 meta-analysis[148,149]. Other authors, however, showed comparable operative times (Table 6)[150-152]. In a recent meta-analysis, Jackson et al[153] analyzed 46 publications and found similar results (OLR 203.9 min vs LLR 203.6 min), although there was great heterogeneity among the studies.
A serious concern in the early days of LLR was the use of pneumoperitoneum with positive pressure. There was a theoretical increased risk of CO2 embolism due to the elevated gradient between the insufflation pressure and the central venous pressure (CVP).
Animal model studies using transesophageal echocardiography demonstrated that gas embolism occurs in more than 2/3 of the animals undergoing LLR. Despite radiologic demonstration of CO2 embolism, this was not associated with any clinical deterioration[21,22,154]. Indeed, the occurrence of gas embolism in clinical practice is anecdotal[91]. CO2 is a highly diffusible gas, which minimizes the risk of embolism as compared to air, and low pneumoperitoneum pressure further reduces its incidence[22,155].
In 2002, Biertho et al[156] reviewed published series of LLR and reported only two cases (1.1%) of possible gas embolism in approximately 200 LLR. In a meta-analysis of comparative studies, Mirnezami et al[149] reported 0.1% incidence of gas embolism.
During major LLR the risk of gas embolism was believed to be higher than for minor hepatectomy due to the wide transection plane with dissection of major hepatic veins and long operative time. However, the occurrence of gas embolism in this scenario is also extremely low. Dagher et al[67] in a multicenter study with 210 cases of major LLR; reported 3 (1.43%) patients that developed gas embolism. However, there was no influence of gas embolism on postoperative morbidity and mortality[67]. Otsuka et al[157] reviewed 477 major hepatectomies from high-volume centers and observed only 3 (0.2%) cases of gas embolism. In recent series, as well as in our experience, no cases of air embolism were observed.
The occurrence of gas embolism has been also related to argon beam coagulation, which increases intra-abdominal pressure leading to an increased risk of gas embolism[158-160]. As argon is not diffusible as CO2, the use of argon beam coagulator during liver transection is not recommended by many experts[59].
The main technical challenge of LLR remains intraoperative hemorrhage during parenchymal transection. Even though intraoperative bleeding rarely occurs, it is difficult to manage in the absence of manual compression.
Some cases of hemorrhagic complications have been reported, mainly related to hepatic veins injuries[161,162], and were managed either laparoscopically or by conversion to laparotomy. Intraoperative bleeding is the main cause of conversion to laparotomy in most series[13,14,81,93,99,163-165].
Major blood loss during liver resection has a direct effect on postoperative course[166] and negatively affects oncological outcomes[116]. Perioperative blood transfusions are associated with a higher rate of recurrence and lower survival after surgical treatment of malignant diseases, especially HCC[10].
Blood loss reported during laparoscopic surgery varies between series, and is directly related to the type and difficulty of LLR[13,14,81,93,99,163-165]. In several meta-analysis of comparative studies intraoperative bleeding tends to be lower at laparoscopic approach than at open resection resulting in decreased requirement for blood transfusion (Table 6)[149,151,153].
Studying patients with malignant diseases (HCC and CRLM), Parks et al[167] showed significantly lower blood loss in the group undergoing LLR than in the group undergoing OLR. Analyzing only patients with HCC, other meta-analyses yielded similar results, with systematic advantage for the group undergoing LLR, with less intraoperative bleeding and lower rates of blood transfusion[117,118,168,169].
The factors responsible for reduced blood loss during LLR are magnified view of the operating field, the positive pressure of the CO2 pneumoperitoneum that avoids retrograde bleeding from hepatic veins, the emergence of new transection devices and adequate inflow and outflow control[170]. In order to address essential steps in bleeding control during LLR, a recent experts’ literature review made some recommendations: Maintenance of pneumoperitoneum between 10-14 mmHg; low CVP (< 5 mmHg); laparoscopy control of inflow and outflow; and surgeons should be experienced with the use of all surgical devices for liver transection and should master laparoscopic suture before starting LLR[170].
The reported conversion rate is in the range of 0%-20%[171], varying mostly according to the indication for LLR. Series on benign disease show conversion rates from nil to 10%[12,17,25,28-33]. Observational comparative studies focused on malignant diseases (Tables 4 and 5) showed conversion rates ranging from 0% to 23.3%[34-58]. However, with surgical expertise the conversion rate can be reduced to < 5% in high-volume expert centers[32,58]. In our experience the overall conversion rate was 6.5%; however, in the last 100 cases, the conversion rate was 3.0%.
The conversion rate is also related to the complexity of the surgical procedure and accumulated experience. In a multicentric review of 210 major hepatectomies conversion rate was 12.4%. To evaluate the effects of learning curve on outcomes, a comparison was made between the first 15 major LLR performed at each center and the subsequent 120 major hepatectomies. Conversion rate (18.8% vs 7.5%, P = 0.0018) was significantly lower in the late group[67]. In patients with cirrhosis reported conversion rates are higher, ranging from 7% to 19.4%[168].
The literature data cited above indicate that LLR is feasible and safe when compared to OLR for both benign and malignant liver lesions. At present, there are 20 meta-analysis summarized on Table 6 comparing the results of LLR and OLR[94,111,117,118,127-130,148-153,167-169,172-174]. Most studies have consistently demonstrated a significantly lower length of stay (LOS) as compared to the open approach. The overall shorter LOS in laparoscopic resection is not only associated with quicker hospital discharge, but an earlier return of bowel activity and lesser requirement of analgesics[148-151]. Rao et al[148] pooled analysis of 32 comparative studies showed significant reduction in LOS (2.96 d, 95%CI: -3.70 to -2.22) and in the time to oral intake (1.33 d, 95%CI: -1.86 to -0.80) in the laparoscopic group.
Nguyen et al[15] found that the overall mortality rate was 0.3% (range 0% to 10%) in 2804 patients operated by LLR until 2008. There were no reported intraoperative deaths. Most common cause of postoperative death was liver failure[15]. Modern series from large volume centers report mortality for LLR in the range of 0% to 2.4%[17,25,32,33,35-49,52-58,81,93,99,164,165]. Jackson et al[153] pooled results of 40 studies comparing mortality rates between LLR and OLR and there were no significant differences between the groups for both in-hospital mortality and postoperative mortality within 30 d of discharge[153].
The comprehensive review of LLR published series by Nguyen et al[15] found an overall morbidity rate was 10.5% (range 0% to 50%). The most common liver-related complication was bile leakage (1.5%) followed by transient hepatic insufficiency (1.0%). The most common general and surgical-related complications were pleural effusions, incisional bleeding and wound infections each with less than 1%[15].
In large series including benign and malignant disease, the overall morbidity rate ranges from 3.2%[175] to 45%. In our series of 214 LLR, morbidity rate was 15% and mortality was 0.5% (one cirrhotic patient died of sepsis). The most common postoperative complications were: Ascitis (15.6%) followed by incisional hernias (9.4%), ileus (9.4%) and pneumonia (9.4%).
Comparative studies showed significant decrease in the complication rate in patients undergoing LLR[149,150,152,153,172]. A meta-analysis published by Mirnezami et al[149] showed a significant decrease in the incidence of liver-specific complications with LLR compared with OLR. Similarly, Jackson et al[153] analyzed 47 studies and demonstrated that patients who underwent LLR had lower postoperative complications rates when compared with OLR. Specifically, minimally invasive approaches had lower rates of wound infections, incisional hernias, and cirrhotic decompensation events.
Regarding malignancies, patients with CRLM who underwent laparoscopic resections also have lower rates of postoperative complications than the open group[127,128,130].
Recently, our group published a series including 30 patients with HCC that underwent LLR. Postoperative complications were observed in 40% of patients (75% grade I by Dindo-Clavien classification) and the mortality rate was 3.3%[176]. A consistent finding among the meta-analysis of LLR for HCC includes reduced complication rates[117,169,173,174]. Xiong et al[117] examined ascites and postoperative liver failure and reported reduced incidences of both. Recently, Morise et al[169] analyzing the subset of patients with known cirrhosis also noted a significant reduced incidence of postoperative ascites and liver failure.
There are concerns that LLR may be associated with increased cost due to laparoscopic equipment[13,74]. Koffron et al[13] showed that the operating room costs for MILS were significantly higher than those of OLR; however, overall costs were reduced due to shorter LOS. Similarly, Polignano et al[177] reported increased disposable instrument costs with LLR. However, these expenses were offset by reduced high dependency unit and ward stay costs, resulting in significantly lower total costs with LLR[177].
In a recent meta-analysis published by Limongelli et al[178], 9 studies were analyzed comparing the costs of patients undergoing LLR (n = 344) vs conventional approach (n = 338). LLR was associated with lower ward stay cost than OLR (2972 USD vs 5291 USD) but costs related to operation (equipment and theatre) were higher in the group of patients undergoing LLR. The total cost was lower in patients managed by LLR (19269 USD) compared to OLR (23419 USD). The same trend of overall cost reduction was observed when the subset of patients undergoing minor LLR was analyzed (total cost: LLR 12720 USD vs OLR 17429 USD).
Regarding major hepatectomies results are contradictory. There is no proven economic benefit related to the laparoscopic procedure when compared to conventional counterparty[13,179-181].
Initial limitations of laparoscopic liver surgery included the fear of unfavorable oncological outcomes[3]. As a new technique, LLR should prove to be non-inferior when compared to the established methods. Pursued oncological results were two-fold: Intraoperative tumor clearance (complete resection with adequate margins) and long-term survival.
As observed for any laparoscopic operation, LLR is performed without tactile feedback along with a limited bi-dimensional field of view. Moreover, as mentioned earlier, the insertion of the laparoscope through or near the umbilicus implicates in a caudal view of the liver[84,98]. Complete resection is the goal for the treatment of hepatic malignancies and the limitations in tactile feedback associated with the modified field of view made surgeons concern about adequate intraoperative oncological results.
The encouraging results of LLR set surgeons to search alternatives to overcome those limitations. LLR performed either with laparoscopic or hand assistance offers the possibility of placing the surgeon’s hand into to the operative field, ruling out the lack of tactile feedback[14]. Moreover, during LLR intraoperative ultrasound should be extensively used, not only to identify occult previously unknown lesions, but most importantly to aid surgical planning in order to obtain clear surgical margins[182].
The best evidence available to date indicates that surgical margins in LLR are as good as in conventional procedures. Comparative studies and meta-analysis have indicated that patients operated with LLR have no increased risk of positive surgical margins[111,117,118,128-130,149,150,168,172-174]. LLR is carried out under a magnified field of view, which implies in augmented perception of operative blood loss and induces surgeons to be more meticulous, especially when employing a new technique[86,115]. Another reason for adequate margins relies on patient selection and surgical planning for laparoscopic cases. Surgery should be extensively planned to include peripheral tumors located away from vascular structures and far from the transection plane[3,14,63,85,93].
Inadequate intraoperative staging, insufficient surgical margins, port-site seeding and peritoneal dissemination were feared outcomes that limited the application of LLR to the treatment of CRLM. Those fears were not confirmed and LLR slowly gained acceptance for operations on CRLM.
The cautious progress demonstrated that results on selected patients proved to be equally good for LLR when compared to conventional operations (Tables 5 and 6)[128,130]. Moreover, LLR increases the chance for future resections once laparoscopy reduces operative adhesions and eases futures interventions[56]. Other technical benefits include the expansion of liver resections to elderly patients and the possibility of synchronous colorectal resections made feasible by a less morbid approach[131,132,183].
HCC is the most common primary liver malignancy and figures as a leading cause of cancer related death[184]. It has a frequent association with chronic liver disease, which implies that management of such tumors comes along with the management of cirrhosis and its complications.
Initial results of laparoscopic operations on cirrhotic patients have shown excellent short-term perioperative outcomes[23,35,66]. One of the intraoperative benefits of LLR is reduced blood loss; perioperative blood transfusions have a negative impact in survival for HCC[116], indicating that laparoscopic resection is a useful tool to improve long-term outcomes.
Comparative and meta-analytical studies took a look into survival rates of LLR and long-term outcomes were superimposed to conventional results (Tables 4 and 6)[111,117,168,173,174]. Thus, LLR is an acceptable option for treating patients with HCC.
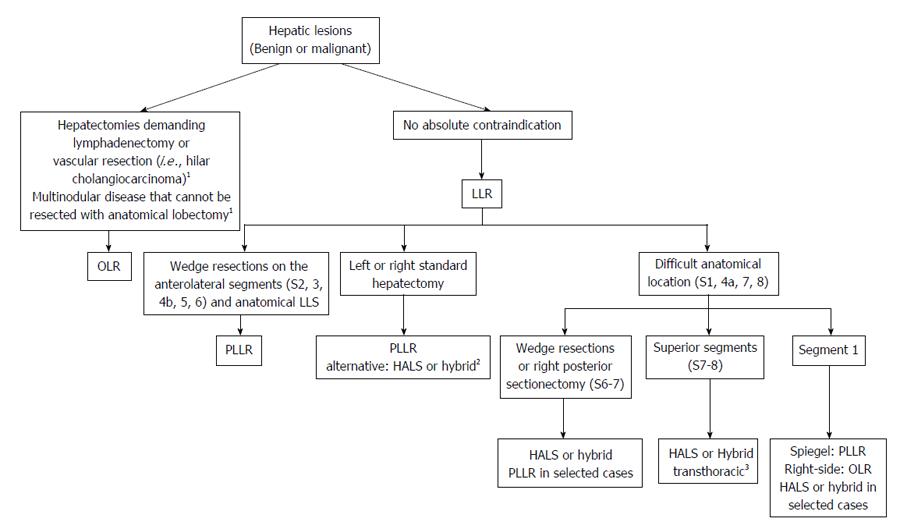
PLLR is the most frequent method of LLR and is mostly applied to less complex operations, such as wedge resections, non-anatomic and anatomic resections on the anterolateral segments[14]. Some expert teams also perform PLLR for major resections and complex procedures such as living donor graft harvesting[19]. HALS offers the advantage of regaining tactile feedback lost with PLLR. It is also helpful in instances were extensive liver mobilization is required, such as posterior sectionectomies and major resections[68]. Hybrid resection associates laparoscopy for liver mobilization with an auxiliary incision for parenchymal transection and specimen removal[14]. Hybrid resections has been reported as a useful tool to increase the frequency of major resections[69]. Figure 1 demonstrates the rationale from the Liver Surgery Unit at University of Sao Paulo Medical School for selecting the best MILS approach for each scenario.
Minor resections were responsible for the successful start of LLR during the 1990’s and still represent one of the major indications of LLR. Especially when located in easily accessible, minor resections should be routinely performed through laparoscopy[14].
Another LLR minor resection that should be routinely performed is LLS[14,33,95]. This resection was the first published successful anatomic LLR and has been extensively studied[8]. Comparative studies have shown that LLS is technically feasible, with superior short-term outcomes and equal long-term oncological results[71,185-187]. Moreover, it is a standardized procedure, allowing reproducibility and training for surgeons initiating their experience with LLR[76,77].
Laparoscopic major resections have been compared to conventional resections and operative results favor LLR on reduced blood loss, shorter length of stay and fewer complications[188,189]. Major resections are feasible procedures but are clearly experts’ job. Published series derive from multi-institutional studies that gather the experience of high-volume centers, were operations are carried out by experienced liver surgery teams[62-64,67].
Access to “non-laparoscopic” segments is difficult once they are located posterior and superior to the liver. Moreover, the postero-superior location demands extensive mobilization in order to bring these segments to the operative field[190]. Mobilization can be toilsome once the right liver should be detached from its ligaments, the diaphragm, retroperitoneum and, sometimes, the IVC.
The perceived technical difficulty to operate on the non-laparoscopic segments has been confirmed in papers that indicate posterior sectionectomies as “major operations” (despite including only two segments), associated with higher conversion rates, higher blood loss, prolonged operative times and narrower surgical margins[61,85,99,100].
Resections on these difficult segments can be performed, but usually demand special techniques to overcome above-mentioned limitations. HALS, laparoscopy-assisted and trans-thoracic port placement are useful strategies applied to difficult resections[103,104,190,191].
MILS has evolved during the past two decades and still moves forward. Robot assisted resections are feasible as reported in case series. Robotic surgery might improve results of LLR once it offers a three-dimensional view and has a greater range of movements, which can be useful for complex resections[24,192,193].
Another perspective for LLR is the association of three-dimensional (3D) image guidance to help surgeons to navigate along the liver anatomy while planning and executing de resection. 3D image simulation appears to be useful for surgical planning and has a high accuracy for predicting surgical margins and liver volumes. Further dynamic applicability of the 3D planning to navigation during operation is expected to improve operative results[194].
Single port operations have been recently incorporated to LLR and anecdotally described. Recent reviews of the scarce available data identified around 30 reported cases. Most cases were highly selected and included small resections, even though major hepatectomy has also been performed. At this point no conclusion or recommendation can be made for single port LLR, further studies are needed to indicate it’s role in LLR[195,196].
LLR has been progressively developed along the past two decades. Despite initial skepticism, improved operative results made LLR incorporated to surgical practice and operations increased in frequency and complexity. However, the expansion of MILS becomes essential when we consider that countries with long-standing tradition in surgery apply laparoscopy to liver surgery in less than 15% of cases. High quality studies allied with high-level surgical training are required to base surgical practice and to disseminate the benefits of MILS to many centers as possible. LLR should be standard practice for anterolateral resections and LLS, major resections are feasible procedures but restricted to experienced centers. Future applicability of new technologies such as robot assistance and image-guided surgery is still under investigation.
P- Reviewer: Ker CG, Mizuguchi I S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, Rotman N, Fagniez PL. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753-762. [PubMed] |
| 2. | Descottes B, Lachachi F, Sodji M, Valleix D, Durand-Fontanier S, Pech de Laclause B, Grousseau D. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg. 2000;232:641-645. [PubMed] |
| 3. | Viganò L, Tayar C, Laurent A, Cherqui D. Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg. 2009;16:410-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Cherqui D. Laparoscopic liver resection. Br J Surg. 2003;90:644-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | D’Albuquerque LA, Herman P. [Laparoscopic hepatectomy: is it a reality?]. Arq Gastroenterol. 2006;43:243-246. [PubMed] |
| 6. | Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956-958. [PubMed] |
| 7. | Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758-761. [PubMed] |
| 8. | Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery. 1996;120:468-475. [PubMed] |
| 9. | Hüscher CG, Lirici MM, Chiodini S, Recher A. Current position of advanced laparoscopic surgery of the liver. J R Coll Surg Edinb. 1997;42:219-225. [PubMed] |
| 10. | Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, Tanaka S, Adachi E, Sugimachi K. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90-97. [PubMed] |
| 12. | Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, Morino M, Bismuth H, Castaing D, Savier E. Laparoscopic liver resection of benign liver tumors. Surg Endosc. 2003;17:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385-392; discussion 392-394. [PubMed] |
| 14. | Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [PubMed] |
| 15. | Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 879] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 16. | Abu Hilal M, Di Fabio F, Abu Salameh M, Pearce NW. Oncological efficiency analysis of laparoscopic liver resection for primary and metastatic cancer: a single-center UK experience. Arch Surg. 2012;147:42-48. [PubMed] |
| 17. | Herman P, Coelho FF, Perini MV, Lupinacci RM, D’Albuquerque LA, Cecconello I. Hepatocellular adenoma: an excellent indication for laparoscopic liver resection. HPB (Oxford). 2012;14:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Doughtie CA, Egger ME, Cannon RM, Martin RC, McMasters KM, Scoggins CR. Laparoscopic hepatectomy is a safe and effective approach for resecting large colorectal liver metastases. Am Surg. 2013;79:566-571. [PubMed] |
| 19. | Cauchy F, Schwarz L, Scatton O, Soubrane O. Laparoscopic liver resection for living donation: where do we stand? World J Gastroenterol. 2014;20:15590-15598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Scatton O, Katsanos G, Boillot O, Goumard C, Bernard D, Stenard F, Perdigao F, Soubrane O. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg. 2015;261:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Schmandra TC, Mierdl S, Hollander D, Hanisch E, Gutt C. Risk of gas embolism in hand-assisted versus total laparoscopic hepatic resection. Surg Technol Int. 2004;12:137-143. [PubMed] |
| 22. | Jayaraman S, Khakhar A, Yang H, Bainbridge D, Quan D. The association between central venous pressure, pneumoperitoneum, and venous carbon dioxide embolism in laparoscopic hepatectomy. Surg Endosc. 2009;23:2369-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol. 2014;20:4892-4899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Tranchart H, Dagher I. Laparoscopic liver resection: a review. J Visc Surg. 2014;151:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Ding G, Cai W, Qin M. Pure Laparoscopic Versus Open Liver Resection in Treatment of Hepatolithiasis Within the Left Lobes: A Randomized Trial Study. Surg Laparosc Endosc Percutan Tech. 2015;25:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Fretland ÅA, Kazaryan AM, Bjørnbeth BA, Flatmark K, Andersen MH, Tønnessen TI, Bjørnelv GM, Fagerland MW, Kristiansen R, Øyri K. Open versus laparoscopic liver resection for colorectal liver metastases (the Oslo-CoMet Study): study protocol for a randomized controlled trial. Trials. 2015;16:73. [PubMed] |
| 27. | van Dam RM, Wong-Lun-Hing EM, van Breukelen GJ, Stoot JH, van der Vorst JR, Bemelmans MH, Olde Damink SW, Lassen K, Dejong CH. Open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery ERAS® programme (ORANGE II-trial): study protocol for a randomised controlled trial. Trials. 2012;13:54. [PubMed] |
| 28. | Katkhouda N, Hurwitz M, Gugenheim J, Mavor E, Mason RJ, Waldrep DJ, Rivera RT, Chandra M, Campos GM, Offerman S. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229:460-466. [PubMed] |
| 29. | Ardito F, Tayar C, Laurent A, Karoui M, Loriau J, Cherqui D. Laparoscopic liver resection for benign disease. Arch Surg. 2007;142:1188-1193; discussion 1193. [PubMed] |
| 30. | Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, De Gendt S, de Hemptinne B. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Abu Hilal M, Di Fabio F, Wiltshire RD, Hamdan M, Layfield DM, Pearce NW. Laparoscopic liver resection for hepatocellular adenoma. World J Gastrointest Surg. 2011;3:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Abu Hilal M, Di Fabio F, Teng MJ, Godfrey DA, Primrose JN, Pearce NW. Surgical management of benign and indeterminate hepatic lesions in the era of laparoscopic liver surgery. Dig Surg. 2011;28:232-236. [PubMed] |
| 33. | Dokmak S, Raut V, Aussilhou B, Ftériche FS, Farges O, Sauvanet A, Belghiti J. Laparoscopic left lateral resection is the gold standard for benign liver lesions: a case-control study. HPB (Oxford). 2014;16:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Belli G, Limongelli P, Fantini C, D’Agostino A, Cioffi L, Belli A, Russo G. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc. 2011;25:3668-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg. 2011;35:2268-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Ker CG, Chen JS, Kuo KK, Chuang SC, Wang SJ, Chang WC, Lee KT, Chen HY, Juan CC. Liver Surgery for Hepatocellular Carcinoma: Laparoscopic versus Open Approach. Int J Hepatol. 2011;2011:596792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 40. | Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One. 2013;8:e72328. [PubMed] |
| 41. | Memeo R, de’Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, Azoulay D. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg. 2014;38:2919-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 42. | Kim SJ, Jung HK, Lee DS, Yun SS, Kim HJ. The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Treat Res. 2014;86:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Ahn KS, Kang KJ, Kim YH, Kim TS, Lim TJ. A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A. 2014;24:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Yamashita Y, Ikeda T, Kurihara T, Yoshida Y, Takeishi K, Itoh S, Harimoto N, Kawanaka H, Shirabe K, Maehara Y. Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single-center experience over a 10-year period. J Am Coll Surg. 2014;219:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Yoon SY, Kim KH, Jung DH, Yu A, Lee SG. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc. 2015;29:2628-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Martin RC, Mbah NA, St Hill R, Kooby D, Weber S, Scoggins CR, Maithel SK. Laparoscopic versus open hepatic resection for hepatocellular carcinoma: improvement in outcomes and similar cost. World J Surg. 2015;39:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Lee JJ, Conneely JB, Smoot RL, Gallinger S, Greig PD, Moulton CA, Wei A, McGilvray I, Cleary SP. Laparoscopic versus open liver resection for hepatocellular carcinoma at a North-American Centre: a 2-to-1 matched pair analysis. HPB (Oxford). 2015;17:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. J Hepatol. 2015;63:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 49. | Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc. 2015;29:2994-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 51. | Abu Hilal M, Underwood T, Zuccaro M, Primrose J, Pearce N. Short- and medium-term results of totally laparoscopic resection for colorectal liver metastases. Br J Surg. 2010;97:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Cannon RM, Scoggins CR, Callender GG, McMasters KM, Martin RC. Laparoscopic versus open resection of hepatic colorectal metastases. Surgery. 2012;152:567-573; discussion 573-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Qiu J, Chen S, Pankaj P, Wu H. Laparoscopic hepatectomy for hepatic colorectal metastases -- a retrospective comparative cohort analysis and literature review. PLoS One. 2013;8:e60153. [PubMed] |
| 54. | Guerron AD, Aliyev S, Agcaoglu O, Aksoy E, Taskin HE, Aucejo F, Miller C, Fung J, Berber E. Laparoscopic versus open resection of colorectal liver metastasis. Surg Endosc. 2013;27:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Kubota Y, Otsuka Y, Tsuchiya M, Katagiri T, Ishii J, Maeda T, Tamura A, Kaneko H. Efficacy of laparoscopic liver resection in colorectal liver metastases and the influence of preoperative chemotherapy. World J Surg Oncol. 2014;12:351. [PubMed] |
| 56. | Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, Smeets P, Brescia A, Rogiers X, de Hemptinne B. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol. 2014;40:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | de’Angelis N, Eshkenazy R, Brunetti F, Valente R, Costa M, Disabato M, Salloum C, Compagnon P, Laurent A, Azoulay D. Laparoscopic versus open resection for colorectal liver metastases: a single-center study with propensity score analysis. J Laparoendosc Adv Surg Tech A. 2015;25:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Hasegawa Y, Nitta H, Sasaki A, Takahara T, Itabashi H, Katagiri H, Otsuka K, Nishizuka S, Wakabayashi G. Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: A comparative analysis of 168 consecutive cases at a single center. Surgery. 2015;157:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 60. | Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 61. | Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery. 2008;144:32-38. [PubMed] |
| 62. | Di Fabio F, Samim M, Di Gioia P, Godeseth R, Pearce NW, Abu Hilal M. Laparoscopic major hepatectomies: clinical outcomes and classification. World J Surg. 2014;38:3169-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Tzanis D, Shivathirthan N, Laurent A, Abu Hilal M, Soubrane O, Kazaryan AM, Ettore GM, Van Dam RM, Lainas P, Tranchart H. European experience of laparoscopic major hepatectomy. J Hepatobiliary Pancreat Sci. 2013;20:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Dagher I, Gayet B, Tzanis D, Tranchart H, Fuks D, Soubrane O, Han HS, Kim KH, Cherqui D, O’Rourke N. International experience for laparoscopic major liver resection. J Hepatobiliary Pancreat Sci. 2014;21:732-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 65. | Soubrane O, Cherqui D, Scatton O, Stenard F, Bernard D, Branchereau S, Martelli H, Gauthier F. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg. 2006;244:815-820. [PubMed] |
| 66. | Soubrane O, Goumard C, Laurent A, Tranchart H, Truant S, Gayet B, Salloum C, Luc G, Dokmak S, Piardi T. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB (Oxford). 2014;16:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Dagher I, O’Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, Lainas P, Laurent A, Nguyen KT, Marvin MR. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 68. | Cardinal JS, Reddy SK, Tsung A, Marsh JW, Geller DA. Laparoscopic major hepatectomy: pure laparoscopic approach versus hand-assisted technique. J Hepatobiliary Pancreat Sci. 2013;20:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Nitta H, Sasaki A, Otsuka Y, Tsuchiya M, Kaneko H, Wakabayashi G. Impact of hybrid techniques on laparoscopic major hepatectomies. J Hepatobiliary Pancreat Sci. 2013;20:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Takahara T, Wakabayashi G, Hasegawa Y, Nitta H. Minimally invasive donor hepatectomy: evolution from hybrid to pure laparoscopic techniques. Ann Surg. 2015;261:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 73. | Kluger MD, Vigano L, Barroso R, Cherqui D. The learning curve in laparoscopic major liver resection. J Hepatobiliary Pancreat Sci. 2013;20:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Nguyen KT, Geller DA. Laparoscopic liver resection--current update. Surg Clin North Am. 2010;90:749-760. [PubMed] |
| 75. | Zhang H, Liu T, Wang Y, Liu HF, Zhang JT, Wu YS, Lei L, Wang HB. Laparoscopic left hepatectomy in swine: a safe and feasible technique. J Vet Sci. 2014;15:417-422. [PubMed] |
| 76. | Khan AZ, Prasad KR, Lodge JP, Toogood GJ. Laparoscopic left lateral sectionectomy: surgical technique and our results from Leeds. J Laparoendosc Adv Surg Tech A. 2009;19:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Hasegawa Y, Nitta H, Sasaki A, Takahara T, Ito N, Fujita T, Kanno S, Nishizuka S, Wakabayashi G. Laparoscopic left lateral sectionectomy as a training procedure for surgeons learning laparoscopic hepatectomy. J Hepatobiliary Pancreat Sci. 2013;20:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 79. | Rau HG, Buttler E, Meyer G, Schardey HM, Schildberg FW. Laparoscopic liver resection compared with conventional partial hepatectomy--a prospective analysis. Hepatogastroenterology. 1998;45:2333-2338. [PubMed] |
| 80. | Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg. 2013;257:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 81. | Cai X, Li Z, Zhang Y, Yu H, Liang X, Jin R, Luo F. Laparoscopic liver resection and the learning curve: a 14-year, single-center experience. Surg Endosc. 2014;28:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 82. | Spampinato MG, Arvanitakis M, Puleo F, Mandala L, Quarta G, Baldazzi G. Assessing the learning curve for totally laparoscopic major-complex liver resections: a single hepatobiliary surgeon experience. Surg Laparosc Endosc Percutan Tech. 2015;25:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Nomi T, Fuks D, Kawaguchi Y, Mal F, Nakajima Y, Gayet B. Learning curve for laparoscopic major hepatectomy. Br J Surg. 2015;102:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 84. | Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 85. | Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg. 2012;256:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 86. | Cannon RM, Saggi B, Buell JF. Evaluation of a laparoscopic liver resection in the setting of cirrhosis. HPB (Oxford). 2014;16:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Farges O, Goutte N, Dokmak S, Bendersky N, Falissard B. How surgical technology translates into practice: the model of laparoscopic liver resections performed in France. Ann Surg. 2014;260:916-921; discussion 921-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Kim Y, Amini N, He J, Margonis GA, Weiss M, Wolfgang CL, Makary M, Hirose K, Spolverato G, Pawlik TM. National trends in the use of surgery for benign hepatic tumors in the United States. Surgery. 2015;157:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Mostaedi R, Milosevic Z, Han HS, Khatri VP. Laparoscopic liver resection: Current role and limitations. World J Gastrointest Oncol. 2012;4:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Kazaryan AM, Røsok BI, Edwin B. Laparoscopic and open liver resection for colorectal metastases: different indications? HPB (Oxford). 2010;12:434; author reply 435. [PubMed] |
| 91. | Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 92. | Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 93. | Gobardhan PD, Subar D, Gayet B. Laparoscopic liver surgery: An overview of the literature and experiences of a single centre. Best Pract Res Clin Gastroenterol. 2014;28:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Rao A, Rao G, Ahmed I. Laparoscopic left lateral liver resection should be a standard operation. Surg Endosc. 2011;25:1603-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Belli G, Gayet B, Han HS, Wakabayashi G, Kim KH, Cannon R, Kaneko H, Gamblin T, Koffron A, Dagher I. Laparoscopic left hemihepatectomy a consideration for acceptance as standard of care. Surg Endosc. 2013;27:2721-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Abu Hilal M, van der Poel MJ, Samim M, Besselink MG, Flowers D, Stedman B, Pearce NW. Laparoscopic liver resection for lesions adjacent to major vasculature: feasibility, safety and oncological efficiency. J Gastrointest Surg. 2015;19:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Gringeri E, Boetto R, Bassi D, D’Amico FE, Polacco M, Romano M, Barbieri S, Feltracco P, Spampinato M, Zanus G. Totally laparoscopic caudate lobe resection: technical aspects and literature review. Surg Laparosc Endosc Percutan Tech. 2014;24:e233-e236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Tomishige H, Morise Z, Kawabe N, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R, Isetani M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg. 2013;5:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Troisi RI, Montalti R, Van Limmen JG, Cavaniglia D, Reyntjens K, Rogiers X, De Hemptinne B. Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB (Oxford). 2014;16:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Kazaryan AM, Røsok BI, Marangos IP, Rosseland AR, Edwin B. Comparative evaluation of laparoscopic liver resection for posterosuperior and anterolateral segments. Surg Endosc. 2011;25:3881-3889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Aikawa M, Miyazawa M, Okamoto K, Toshimitsu Y, Okada K, Ueno Y, Yamaguchi S, Koyama I. Thoracoscopic hepatectomy for malignant liver tumor. Surg Endosc. 2014;28:314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci. 2014;21:E65-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Krüger JA, Coelho FF, Perini MV, Herman P. Laparoscopic transthoracic liver resection. Arq Bras Cir Dig. 2014;27:288-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Ogiso S, Conrad C, Araki K, Nomi T, Anil Z, Gayet B. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg. 2015;262:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 105. | Cai X, Wang Y, Yu H, Liang X, Peng S. Laparoscopic hepatectomy for hepatolithiasis: a feasibility and safety study in 29 patients. Surg Endosc. 2007;21:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Agrawal S, Agarwal S, Arnason T, Saini S, Belghiti J. Management of Hepatocellular Adenoma: Recent Advances. Clin Gastroenterol Hepatol. 2015;13:1221-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 107. | Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 108. | Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210-215. [PubMed] |
| 109. | Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27:2592-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 110. | Hu BS, Chen K, Tan HM, Ding XM, Tan JW. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4725-4728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Fancellu A, Rosman AS, Sanna V, Nigri GR, Zorcolo L, Pisano M, Melis M. Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J Surg Res. 2011;171:e33-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Marubashi S, Gotoh K, Akita H, Takahashi H, Sugimura K, Miyoshi N, Motoori M, Kishi K, Noura S, Fujiwara Y. Analysis of Recurrence Patterns After Anatomical or Non-anatomical Resection for Hepatocellular Carcinoma. Ann Surg Oncol. 2015;22:2243-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Marubashi S, Gotoh K, Akita H, Takahashi H, Ito Y, Yano M, Ishikawa O, Sakon M. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 114. | Kang CM, Choi GH, Kim DH, Choi SB, Kim KS, Choi JS, Lee WJ. Revisiting the role of nonanatomic resection of small (< or = 4 cm) and single hepatocellular carcinoma in patients with well-preserved liver function. J Surg Res. 2010;160:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Kaneko H, Tsuchiya M, Otsuka Y, Yajima S, Minagawa T, Watanabe M, Tamura A. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg. 2009;16:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 116. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 117. | Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, Sutton R, Liu XB, Hu WM. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6657-6668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 118. | Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2011;56:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 119. | Laurent A, Tayar C, Andréoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 120. | Perini MV, Starkey G, Fink MA, Bhandari R, Muralidharan V, Jones R, Christophi C. From minimal to maximal surgery in the treatment of hepatocarcinoma: A review. World J Hepatol. 2015;7:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Belli G, Cioffi L, Fantini C, D’Agostino A, Russo G, Limongelli P, Belli A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc. 2009;23:1807-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Dagher I, Belli G, Fantini C, Laurent A, Tayar C, Lainas P, Tranchart H, Franco D, Cherqui D. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg. 2010;211:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 123. | Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, Ohira G, Nakajima T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2013;20:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 124. | Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 413] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 125. | Abdalla EK, Bauer TW, Chun YS, D’Angelica M, Kooby DA, Jarnagin WR. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB (Oxford). 2013;15:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 126. | Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, Marvin M, Ravindra KV, Mejia A, Lainas P. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 127. | Wei M, He Y, Wang J, Chen N, Zhou Z, Wang Z. Laparoscopic versus open hepatectomy with or without synchronous colectomy for colorectal liver metastasis: a meta-analysis. PLoS One. 2014;9:e87461. [PubMed] |
| 128. | Luo LX, Yu ZY, Bai YN. Laparoscopic hepatectomy for liver metastases from colorectal cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2014;24:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Zhou Y, Xiao Y, Wu L, Li B, Li H. Laparoscopic liver resection as a safe and efficacious alternative to open resection for colorectal liver metastasis: a meta-analysis. BMC Surg. 2013;13:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 130. | Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery. 2015;157:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 131. | Nomi T, Fuks D, Kawaguchi Y, Mal F, Nakajima Y, Gayet B. Laparoscopic major hepatectomy for colorectal liver metastases in elderly patients: a single-center, case-matched study. Surg Endosc. 2015;29:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Tranchart H, Diop PS, Lainas P, Pourcher G, Catherine L, Franco D, Dagher I. Laparoscopic major hepatectomy can be safely performed with colorectal surgery for synchronous colorectal liver metastasis. HPB (Oxford). 2011;13:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Hu MG, Ou-yang CG, Zhao GD, Xu DB, Liu R. Outcomes of open versus laparoscopic procedure for synchronous radical resection of liver metastatic colorectal cancer: a comparative study. Surg Laparosc Endosc Percutan Tech. 2012;22:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 134. | Lin E, Sarmiento JM. Laparoscopic extended right hepatectomy, portal lymphadenectomy, and hepaticojejunostomy for hilar cholangiocarcinoma. J Laparoendosc Adv Surg Tech A. 2014;24:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Yu H, Wu SD, Chen DX, Zhu G. Laparoscopic resection of Bismuth type I and II hilar cholangiocarcinoma: an audit of 14 cases from two institutions. Dig Surg. 2011;28:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 136. | Alkhalili E, Berber E. Laparoscopic liver resection for malignancy: a review of the literature. World J Gastroenterol. 2014;20:13599-13606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 137. | Thenappan A, Jha RC, Fishbein T, Matsumoto C, Melancon JK, Girlanda R, Shetty K, Laurin J, Plotkin J, Johnson L. Liver allograft outcomes after laparoscopic-assisted and minimal access live donor hepatectomy for transplantation. Am J Surg. 2011;201:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, Branchereau S, Chardot C, Gauthier F, Fagniez PL. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359:392-396. [PubMed] |
| 139. | McPhail MJ, Scibelli T, Abdelaziz M, Titi A, Pearce NW, Abu Hilal M. Laparoscopic versus open left lateral hepatectomy. Expert Rev Gastroenterol Hepatol. 2009;3:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Troisi RI, Wojcicki M, Tomassini F, Houtmeyers P, Vanlander A, Berrevoet F, Smeets P, Van Vlierberghe H, Rogiers X. Pure laparoscopic full-left living donor hepatectomy for calculated small-for-size LDLT in adults: proof of concept. Am J Transplant. 2013;13:2472-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 141. | Samstein B, Cherqui D, Rotellar F, Griesemer A, Halazun KJ, Kato T, Guarrera J, Emond JC. Totally laparoscopic full left hepatectomy for living donor liver transplantation in adolescents and adults. Am J Transplant. 2013;13:2462-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 142. | Marubashi S, Wada H, Kawamoto K, Kobayashi S, Eguchi H, Doki Y, Mori M, Nagano H. Laparoscopy-assisted hybrid left-side donor hepatectomy. World J Surg. 2013;37:2202-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant. 2013;13:2467-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 144. | Makki K, Chorasiya VK, Sood G, Srivastava PK, Dargan P, Vij V. Laparoscopy-assisted hepatectomy versus conventional (open) hepatectomy for living donors: when you know better, you do better. Liver Transpl. 2014;20:1229-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 145. | Ha TY, Hwang S, Ahn CS, Kim KH, Moon DB, Song GW, Jung DH, Park GC, Namgoong JM, Park CS. Role of hand-assisted laparoscopic surgery in living-donor right liver harvest. Transplant Proc. 2013;45:2997-2999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 146. | Rotellar F, Pardo F, Benito A, Martí-Cruchaga P, Zozaya G, Lopez L, Hidalgo F, Sangro B, Herrero I. Totally laparoscopic right-lobe hepatectomy for adult living donor liver transplantation: useful strategies to enhance safety. Am J Transplant. 2013;13:3269-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 147. | Berardi G, Tomassini F, Troisi RI. Comparison between minimally invasive and open living donor hepatectomy: A systematic review and meta-analysis. Liver Transpl. 2015;21:738-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 148. | Rao A, Rao G, Ahmed I. Laparoscopic or open liver resection? Let systematic review decide it. Am J Surg. 2012;204:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 149. | Mirnezami R, Mirnezami AH, Chandrakumaran K, Abu Hilal M, Pearce NW, Primrose JN, Sutcliffe RP. Short- and long-term outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB (Oxford). 2011;13:295-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 150. | Croome KP, Yamashita MH. Laparoscopic vs open hepatic resection for benign and malignant tumors: An updated meta-analysis. Arch Surg. 2010;145:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 151. | Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, Antoniou A. Laparoscopic versus open hepatic resections for benign and malignant neoplasms--a meta-analysis. Surgery. 2007;141:203-211. [PubMed] |
| 152. | Mizuguchi T, Kawamoto M, Meguro M, Shibata T, Nakamura Y, Kimura Y, Furuhata T, Sonoda T, Hirata K. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today. 2011;41:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Jackson NR, Hauch A, Hu T, Buell JF, Slakey DP, Kandil E. The safety and efficacy of approaches to liver resection: a meta-analysis. JSLS. 2015;19:e2014.00186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 154. | Fors D, Eiriksson K, Arvidsson D, Rubertsson S. Gas embolism during laparoscopic liver resection in a pig model: frequency and severity. Br J Anaesth. 2010;105:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 155. | Bazin JE, Gillart T, Rasson P, Conio N, Aigouy L, Schoeffler P. Haemodynamic conditions enhancing gas embolism after venous injury during laparoscopy: a study in pigs. Br J Anaesth. 1997;78:570-575. [PubMed] |
| 157. | Otsuka Y, Katagiri T, Ishii J, Maeda T, Kubota Y, Tamura A, Tsuchiya M, Kaneko H. Gas embolism in laparoscopic hepatectomy: what is the optimal pneumoperitoneal pressure for laparoscopic major hepatectomy? J Hepatobiliary Pancreat Sci. 2013;20:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 158. | Palmer M, Miller CW, van Way CW, Orton EC. Venous gas embolism associated with argon-enhanced coagulation of the liver. J Invest Surg. 1993;6:391-399. [PubMed] |
| 159. | Min SK, Kim JH, Lee SY. Carbon dioxide and argon gas embolism during laparoscopic hepatic resection. Acta Anaesthesiol Scand. 2007;51:949-953. [PubMed] |
| 160. | Ikegami T, Shimada M, Imura S, Nakamura T, Kawahito S, Morine Y, Kanemura H, Hanaoka J. Argon gas embolism in the application of laparoscopic microwave coagulation therapy. J Hepatobiliary Pancreat Surg. 2009;16:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 161. | Dagher I, Proske JM, Carloni A, Richa H, Tranchart H, Franco D. Laparoscopic liver resection: results for 70 patients. Surg Endosc. 2007;21:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 162. | Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 163. | Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 164. | Long TC, Bac NH, Thuan ND, Dat le T, Viet DQ, Chuong le CH. Laparoscopic liver resection: 5-year experience at a single center. Surg Endosc. 2014;28:796-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 165. | Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, Brock G, McMasters KM. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 166. | de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259-264. [PubMed] |
| 167. | Parks KR, Kuo YH, Davis JM, O’ Brien B, Hagopian EJ. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB (Oxford). 2014;16:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 168. | Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol. 2014;20:8274-8281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 169. | Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci. 2015;22:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 170. | Tranchart H, O’Rourke N, Van Dam R, Gaillard M, Lainas P, Sugioka A, Wakabayashi G, Dagher I. Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pancreat Sci. 2015;22:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 171. | Edwin B, Nordin A, Kazaryan AM. Laparoscopic liver surgery: new frontiers. Scand J Surg. 2011;100:54-65. [PubMed] |
| 172. | Rao A, Rao G, Ahmed I. Laparoscopic vs. open liver resection for malignant liver disease. A systematic review. Surgeon. 2012;10:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 173. | Li N, Wu YR, Wu B, Lu MQ. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2012;42:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 174. | Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 175. | Cai XJ, Yu H, Liang X, Wang YF, Zheng XY, Huang DY, Peng SY. Laparoscopic hepatectomy by curettage and aspiration. Experiences of 62 cases. Surg Endosc. 2006;20:1531-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 176. | Herman P, Perini MV, Coelho FF, Kruger JA, Lupinacci RM, Fonseca GM, Lopes Fde L, Cecconello I. Laparoscopic resection of hepatocellular carcinoma: when, why, and how? A single-center experience. J Laparoendosc Adv Surg Tech A. 2014;24:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 177. | Polignano FM, Quyn AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc. 2008;22:2564-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 178. | Limongelli P, Vitiello C, Belli A, Pai M, Tolone S, Del Genio G, Brusciano L, Docimo G, Habib N, Belli G. Costs of laparoscopic and open liver and pancreatic resection: a systematic review. World J Gastroenterol. 2014;20:17595-17602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 179. | Abu Hilal M, Di Fabio F, Syed S, Wiltshire R, Dimovska E, Turner D, Primrose JN, Pearce NW. Assessment of the financial implications for laparoscopic liver surgery: a single-centre UK cost analysis for minor and major hepatectomy. Surg Endosc. 2013;27:2542-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 180. | Medbery RL, Chadid TS, Sweeney JF, Knechtle SJ, Kooby DA, Maithel SK, Lin E, Sarmiento JM. Laparoscopic vs open right hepatectomy: a value-based analysis. J Am Coll Surg. 2014;218:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 181. | Cannon RM, Scoggins CR, Callender GG, Quillo A, McMasters KM, Martin RC. Financial comparison of laparoscopic versus open hepatic resection using deviation-based cost modeling. Ann Surg Oncol. 2013;20:2887-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 182. | Viganò L, Ferrero A, Amisano M, Russolillo N, Capussotti L. Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br J Surg. 2013;100:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 183. | Inoue A, Uemura M, Yamamoto H, Hiraki M, Naito A, Ogino T, Nonaka R, Nishimura J, Wada H, Hata T. Short-term outcomes of simultaneous laparoscopic colectomy and hepatectomy for primary colorectal cancer with synchronous liver metastases. Int Surg. 2014;99:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 184. | Jarnagin W, Chapman WC, Curley S, D’Angelica M, Rosen C, Dixon E, Nagorney D. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 185. | Abu Hilal M, McPhail MJ, Zeidan B, Zeidan S, Hallam MJ, Armstrong T, Primrose JN, Pearce NW. Laparoscopic versus open left lateral hepatic sectionectomy: A comparative study. Eur J Surg Oncol. 2008;34:1285-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 186. | Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236-242. [PubMed] |
| 187. | Aldrighetti L, Pulitanò C, Catena M, Arru M, Guzzetti E, Casati M, Comotti L, Ferla G. A prospective evaluation of laparoscopic versus open left lateral hepatic sectionectomy. J Gastrointest Surg. 2008;12:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 188. | Dagher I, Di Giuro G, Dubrez J, Lainas P, Smadja C, Franco D. Laparoscopic versus open right hepatectomy: a comparative study. Am J Surg. 2009;198:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 189. | Abu Hilal M, Di Fabio F, Teng MJ, Lykoudis P, Primrose JN, Pearce NW. Single-centre comparative study of laparoscopic versus open right hepatectomy. J Gastrointest Surg. 2011;15:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 190. | Herman P, Krüger J, Lupinacci R, Coelho F, Perini M. Laparoscopic bisegmentectomy 6 and 7 using a Glissonian approach and a half-Pringle maneuver. Surg Endosc. 2013;27:1840-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 191. | Chiow AK, Lewin J, Manoharan B, Cavallucci D, Bryant R, O’Rourke N. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford). 2015;17:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 192. | Montalti R, Patriti A, Troisi RI. Robotic Versus Laparoscopic Hepatectomy: What Is the Best Minimally Invasive Approach? Ann Surg. 2015;262:e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 193. | Spampinato MG, Coratti A, Bianco L, Caniglia F, Laurenzi A, Puleo F, Ettorre GM, Boggi U. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc. 2014;28:2973-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 194. | Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat Sci. 2015;22:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 195. | Chang SK, Lee KY. Therapeutic advances: single incision laparoscopic hepatopancreatobiliary surgery. World J Gastroenterol. 2014;20:14329-14337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 196. | Gkegkes ID, Iavazzo C. Single incision laparoscopic hepatectomy: A systematic review. J Minim Access Surg. 2014;10:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 197. | de’Angelis N, Memeo R, Calderaro J, Felli E, Salloum C, Compagnon P, Luciani A, Laurent A, Cherqui D, Azoulay D. Open and laparoscopic resection of hepatocellular adenoma: trends over 23 years at a specialist hepatobiliary unit. HPB (Oxford). 2014;16:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 198. | Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi Y, Lee HW. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc. 2014;28:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |