Published online Sep 27, 2015. doi: 10.4240/wjgs.v7.i9.196
Peer-review started: May 20, 2015
First decision: July 10, 2015
Revised: July 15, 2015
Accepted: August 10, 2015
Article in press: August 11, 2015
Published online: September 27, 2015
Processing time: 137 Days and 7.7 Hours
AIM: To establish the association between lymph node involvement and the response to neoadjuvant therapy in locally advanced rectal cancer.
METHODS: Data of 130 patients with mid and low locally advanced rectal adenocarcinoma treated with neoadjuvant chemoradiation followed by radical surgery over a 5-year period were reviewed. Tumor staging was done by endorectal ultrasound and/or magnetic resonance imaging. Tumor response to neoadjuvant therapy was determined by T-downstaging and tumor regression grading (TRG). Pathologic complete response (pCR) is defined as the absence of tumor cells in the surgical specimen (ypT0N0). The varying degrees TRG were classified according to Mandard’s scoring system. The evaluation of the response is based on the comparison between previous clinico-radiological staging and the results of pathological evaluation. χ2 and Spearman’s correlation tests were used for the comparison of variables.
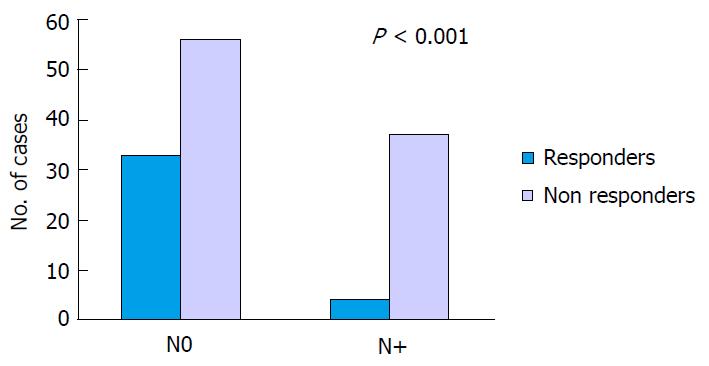
RESULTS: Pathologic complete response (pCR, ypT0N0, TRG1) was observed in 19 cases (14.6%), and other 18 (13.8%) had only very few residual malignant cells in the rectal wall (TRG2). T-downstaging was found in 63 (48.5%). Mean lymph node retrieval was 9.4 (range 0-38). In 37 cases (28.5%) more than 12 nodes were identified in the surgical specimen. Preoperative lymph node involvement was seen in 77 patients (59.2%), 71 N1 and 6 N2. Postoperative lymph node involvement was observed in 41 patients (31.5%), 29 N1 and 12 N2, while the remaining 89 were N0 (68.5%). In relation to ypT stage, we found nodal involvement of 9.4% in ypT0-1, 22.2% in ypT2 and 43.7% in ypT3-4. Of the 37 patients considered “responders” to neoadjuvant therapy (TRG1 and 2), there were only 4 N+ (10.8%) and the remainder N0 (89.2%). In the “non responders” group (TRG 3, 4 and 5), 37 cases were N+ (39.8%) and 56 (60.2%) were N0 (P < 0.001).
CONCLUSION: Response to neoadjuvant chemoradiation in rectal cancer is associated with lymph node involvement.
Core tip: The treatment of rectal cancer has evolved significantly in recent decades. The response of the primary tumor to neoadjuvant therapy, measured by tumor regression grading, seems to be a good prognostic factor, although this relationship is controversial. One of the most important prognostic factors is lymph node stage, but its relationship with the response to neoadyuvant therapy has not been studied extensively. In our series the response is correlated with lymph node involvement in the surgical specimens. Tumor regression grading score could therefore have clinical implications in the future in order to provide tailored therapies.
- Citation: García-Flórez LJ, Gómez-Álvarez G, Frunza AM, Barneo-Serra L, Fresno-Forcelledo MF. Response to chemoradiotherapy and lymph node involvement in locally advanced rectal cancer. World Journal of Gastrointestinal Surgery 2015; 7(9): 196-202
- URL: https://www.wjgnet.com/1948-9366/full/v7/i9/196.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i9.196
Colorectal cancer is one of the most common tumors worldwide, both in males and females, with an estimated 600000 deaths per year[1]. About 70% are located in the colon and 30% in the rectum. The treatment of rectal cancer (RC) has evolved significantly in recent decades. Neoadjuvant therapy with chemoradiation (CRT) improves local control and reduces toxicity compared to postoperative therapies. Sauer et al[2] showed that neoadjuvant CRT is superior in terms of local recurrence (LR) and acute toxicity. Around 60% of these patients experience some degree of tumor regression, but only a minor percentage will show pathologic complete response (pCR)[3].
According to data from the German Rectal Cancer Study Group[4], pCR is associated to better local control, lower risk of distant metastasis and better diseasefree survival. The response of the primary tumor to neoadjuvant therapy, measured by tumor regression grading (TRG) seems to be a good prognostic factor, however this relationship is controversial. In some studies no association with survival was found[5,6], whilst in others it was[7,8]. One of the most important prognostic factors is lymph node stage[9], but its relationship with the response to neoadyuvant therapy has not been studied extensively.
The aim of this study was to establish the relationship between lymph node involvement and the response to neoadjuvant CRT in locally advanced RC.
Data of patients with mid and low locally advanced rectal adenocarcinoma treated with neoadjuvant CRT followed by radical surgery in the University Central Hospital of Asturias over a 5 year period were reviewed. Rectal location is divided into low (2 to 6 cm from anal verge) and mid rectum (7 to 12 cm) measured by rigid proctoscope or magnetic resonance imaging (MRI). Locally advanced RC is defined as a tumor extending beyond the rectal wall (T3-4) or with lymph node involvement (N+), according to the TNM classification of the UICC[10], based on clinical and radiological criteria. Patients with skin or anal cancer, stage T1-2N0 RC, distal margin in upper rectum, with no completion of CRT or with previous pelvic radiotherapy were excluded of the study. Also excluded were those with no record of diagnostic endoscopic biopsy or those with no radical surgery. All patients received long course radiotherapy (45-50.4 Gy) with 5-FU based chemotherapy followed by radical surgery with total mesorectal excision (TME) after a mean of 7 wk interval.
Morphologic evaluation of the surgical specimens was carried out by two experienced pathologist with no knowledge of other clinical data. The evaluation of the response to neoadjuvant CRT is based on the comparison between previous clinico-radiological staging and the results of pathological evaluation, measuring T-downstaging and TRG. PCR is defined as the absence of tumor cells in the surgical specimen (ypT0N0). T-downstaging was evidenced by TNM staging and is defined as the reduction of at least one T level measured initially by endorectal ultrasound and/or pelvic MRI and finally by pathological evaluation. The varying degrees of TRG were classified according to Mandard et al[11] scoring system.
For the statistical analysis the software SPSS Statistics v21 was used. Two groups were established: “Responders”, including TRG1 and 2, and “non responders”, including TRG3, 4 and 5. χ2 and Spearman’s correlation tests were used for the comparison of variables. A P below 0.05 was considered significant. The statistical review of the study was performed by an expert in biomedical statistics.
A sample of 130 patients who met the study criteria was included (Table 1). All patients received full treatment with long cycle radiotherapy (45-50.4 Gy) and 5-FU based chemotherapy (oral capecitabine) followed by radical surgery.
| n (%) | ||
| Age | Mean | 67.4 ± 10.6 |
| Range | 42-86 | |
| Gender | Male | 87 (66.9) |
| Female | 43 (33.1) | |
| Tumor location | Mid rectum | 75 (57.7) |
| Low rectum | 55 (42.3) | |
| Tumor differentiation | Well | 68 (52.3) |
| Moderate | 53 (40.8) | |
| Poor | 9 (6.9) | |
| Staging method | Endorectal ultrasound | 119 (91.5) |
| Magnetic resonance imaging | 47 (36.2) | |
| Radiotherapy | 45 Gy | 84 (64.6) |
| 50.4 Gy | 46 (35.4) | |
| Interval to surgery | Mean | 7.1 ± 1.1 |
| Range | 5-12 | |
| Surgical procedures | Low anterior resection | 55 (42.3) |
| Abdominoperineal resection | 47 (36.2) | |
| Hartmann procedure | 25 (19.2) | |
| Total proctocolectomy | 3 (2.3) |
For staging at baseline, endorectal ultrasound was available in 119 cases and pelvic MRI in 47. In early years of the study, the main staging method was ultrasound. Pelvic MRI is commonly used in recent years (Table 2). In case of disagreement between the two methods (10 cases), MRI was preferably considered. The ypTN (postoperative) staging is showed in Table 3.
| n | % | |
| Pelvic MRI (n = 47) | ||
| T3N1 | 21 | 44.6 |
| T3N0 | 12 | 25.5 |
| T3N2 | 4 | 8.5 |
| T4N0 | 3 | 6.4 |
| T4N1 | 3 | 6.4 |
| T2N1 | 2 | 4.3 |
| T4N2 | 2 | 4.3 |
| ERUS (n = 119) | ||
| T3N1 | 53 | 44.6 |
| T3N0 | 50 | 42 |
| T4N1 | 9 | 7.6 |
| T4N0 | 3 | 2.5 |
| T2N1 | 3 | 2.5 |
| T3N2 | 1 | 0.8 |
| ypTN | n | % |
| T0N0 | 19 | 14.6 |
| T1N0 | 10 | 7.7 |
| T2N0 | 21 | 16.2 |
| T3N0 | 38 | 29.2 |
| T4N0 | 2 | 1.5 |
| T0N1 | 3 | 2.3 |
| T2N1 | 6 | 4.6 |
| T3N1 | 16 | 12.3 |
| T4N1 | 3 | 2.3 |
| T3N2 | 11 | 8.5 |
| T4N2 | 1 | 0.8 |
The result of TRG is included in Table 4. Complete response (pCR, ypT0N0, TRG1) was observed in 19 cases (14.6%), and other 18 (13.8%) had only very few residual malignant cells in the rectal wall (TRG2). These two groups were considered “responders” to neoadjuvant therapy. T-downstaging was seen in 63 patients (48.5%) and progression of tumor stage only in one case.
| TRG | n | % |
| 1 | 19 | 14.6 |
| 2 | 18 | 13.9 |
| 3 | 39 | 30 |
| 4 | 41 | 31.5 |
| 5 | 13 | 10 |
Mean lymph node retrieval was 9.4 (range 0-38). In 37 cases (28.5%) more than 12 nodes were identified in the surgical specimen. Preoperative lymph node involvement was seen in 77 patients (59.2%), 71 N1 and 6 N2. Postoperative lymph node involvement was observed in 41 patients (31.5%), 29 N1 and 12 N2, while the remaining 89 were N0 (68.5%). In relation to ypT stage, we found nodal involvement of 9.4% in ypT0-1, 22.2% in ypT2 and 43.7% in ypT3-4.
Of the 37 patients considered “responders” to neoadjuvant therapy (TRG1 and 2), there were only 4 N+ (10.8%) and the remainder N0 (89.2%). In the “non responders” group, 37 cases were N+ (39.8%) and 56 (60.2%) were N0 (P < 0.001) (Figure 1).
Conventional treatment for locally advanced, clinically resectable (T3-4 and/or N+) tumors is neoadjuvant CRT followed by radical surgery. Our ability to identify the N+ is limited, which leads to potentially overtreat 15%-20% of patients, as the German Trial shows[2], or undertreat 20%-30%. For N stage, both endorectal ultrasound and MRI have similar low sensitivity and specificity rates. Nonetheless, MRI is preferred for N-stage assessment because it allows the evaluation of the whole mesorectum. With radiological imaging advances we have progressed in the identification of adjuvant and neoadjuvant therapy needs. High resolution pelvic MRI with expert radiologist interpretation would help us to select patients that will be correctly treated with just surgery[12]. We are now using MRI to be selective and only irradiate those with a big volume, threatened mesorectal fascia, significant N+ or those with signs of venous invasion. In this line, the prospect trial is investigating the possibility of selectively eliminating the use of neoadjuvant radiotherapy in patients with upper and mid RC[13].
Response rates to CRT are highly variable. Approximately 15%-40% are resistant, while 5%-35% show a pCR. Our results are in that line. Pathological stage and TRG have a significant prognostic impact. Several studies link the TRG with disease-free survival but only pCR is clearly correlated[7,14]. TRG has been studied extensively. Rödel et al[4] analyzed 385 cases and found significant differences when grouped TRG 2 and 3, but not when stratified by pathological stage, giving doubts about the exact significance of this factor. Losi et al[15] found differences in 106 patients only when grouped TRG 3 and 4, although there was a trend towards improved disease-free survival when TRG was stratified by pathological stage. Moreno García et al[16] found that both disease-free survival and overall survival significantly improved with increased TRG. However, the correlation of the response to neoadjuvant CRT and LR and survival is still controversial[17,18].
In our series, we found a 14.6% of pCR. Patients with pCR have a better prognosis, with excellent local control and disease-free survival, regardless of previous TN stage[4,15,19-22]. Capirci et al[23] reviewed a large series of 566 cases with pCR in 61 centers and found better prognosis in this group. A number of groups are currently studying the possibility of treating the RC when a complete clinical response is achieved with local excision or observation (wait and see approach). Because approximately 40%-50% of patients treated with CRT will be ypT0-2 stage and a 10%-20% will be pCR (in our series 45.4% and 14.5% respectively), these preservation strategies of the rectum may have a potential application in many patients. However, there is a weak correlation between clinical and pathological response. Complete pathologic response cannot be accurately identified by clinical, endoscopic or radiological examination and, in most cases, is carried out with subjective exploration data[24-26]. One of the main questions that arise when performing local surgery is the nodal status. The incidence of lymph node involvement after neoadjuvant therapy varies. Some studies indicate differences in response between the tumor and the mesorectal lymph nodes[24,27]. The risk of lymph node involvement in patients treated with CRT and ypT0 tumors is low, but increases significantly with the degree of tumor penetration if any residual neoplastic cells remain in the rectal wall[28]. The risk of nodal metastasis in ypT0-1 is about 7%, compared to 30% for ypT2-4 (range 23%-37%). Read et al[29] found 3.5% involvement in T0-1, 23% in T2 and 51.5% in T3-4. Zmora et al[30] observed a higher incidence in T0-1, 12.1%. Park et al[31] found similar data: ypT0 9.1%, ypT1 17.1%, ypT2 20.8%. In our series we found 9.4% nodal involvement in ypT0-1, 22.2% in ypT2 and 43.7% in ypT3-4. Therefore, the identification of predictive criteria related both the primary tumor and lymph nodes seems to be important to select patients for local surgery, because we must not forget that radical surgical resection with TME, gold standard to compare with other alternatives, is associated with very good oncologic outcomes. In line with our study, Berho et al[32] found correlation between postoperative N stage and TRG, suggesting that neoadjuvant therapy should have a positive impact on overall survival. This study shows the low incidence of lymph node metastasis (14.2%) in good responders, findings similar to ours, where the percentage in TRG1 and 2 patients was 10.8%.
Our data confirms the association between the response to neoadjuvant therapy and lymph node involvement in RC[29,32,33]. Some studies have shown a relationship between good response to CRT and survival, suggesting that oncologic outcomes are more related to postoperative TNM stage, so TRG may be emerging as an independent prognostic factor[15,22,34]. The correlation with ypT stage strengthens this hypothesis. Dhadda et al[35] (n = 158) concluded that Mandard’s scoring system is an independent prognostic factor predicting long-term outcomes. This index has already shown association with prognosis in esophageal cancer patients after CRT[11]. The authors propose its use in assessing the adjuvant therapy. Patients with TRG1-2 would be those with tumors sensitive to 5-FU therapy, while TRG3-5 or with positive nodes have worse prognosis and will require more intensive therapies.
The number of positive nodes is related not only with vascular invasion, but also with the reported number, which varies depending on factors related to the patient (age, sex, body mass index), the tumor (size, stage, grade), and the experience of the surgeon and the pathologist[36]. In our series the average nodes retrieval in the surgical specimen was 9.4. Although the American Joint Committee on Cancer recommends a minimum of 12 nodes for a correct staging, the number of isolated nodes in RC without treatment ranges from 9 to 13 and in patients with neoadjuvant therapy is usually lower[37-39], in part because of the depletion due to treatment and fibrosis, which makes the nodes smaller and more difficult to identify. The significance of this issue is unclear. Some authors consider it a marker of better response and is associated with a higher rate of pCR[26]. Marks et al[40] (n = 176) found only 28% of patients treated with CRT followed by TME in which more than 12 lymph nodes were identified in the resected specimen. Similar data were observed in a study by Govindarajan et al[41] (n = 429), where the average retrieved nodes was 10% and 63% of cases were under 12. In our series, only in 28.5% of cases more than 12 lymph nodes were identified. The inability to study more than 12 nodes is not associated to a worse prognosis in RC. Habr-Gama et al[42] showed that patients with no identifiable lymph nodes in the resected proctectomy specimens after CRT have excellent oncologic outcomes similar to those with ypN0 stage. Sprenger et al[43] have managed to increase, by intensive pathological examination, the number of identified lymph nodes and the incidence of N+, often with the presence of micrometastasis, although with no prognostic significance. Newer therapy strategies could have an impact in the near future[44].
In conclusion, in our series the response to neoadjuvant CRT in locally advanced rectal cancer is correlated with lymph node involvement in the surgical specimens. TRG therefore could have clinical implications in the future in order to provide tailored therapies.
The authors would like to thank the members of the Hospital Universitario Central de Asturias Human Tissue Biobank for their technical support and Ángela García-González for the statistical review of the study.
Neoadjuvant therapy with chemoradiation (CRT) improves local control and reduces toxicity compared to postoperative therapies. CRT is superior in terms of local recurrence and acute toxicity. Around 60% of these patients experience some degree of tumor regression, but only a minor percentage will show pathologic complete response. The response of the primary tumor to neoadjuvant therapy, measured by tumor regression grading seems to be a good prognostic factor, nevertheless this relationship is controversial.
One of the most important prognostic factors is lymph node stage, but its relationship with the response to neoadyuvant therapy has not been studied extensively.
This is a study to establish the relationship between lymph node involvement and the response to neoadjuvant CRT in locally advanced rectal cancer.
In the series the response to neoadjuvant CRT in locally advanced rectal cancer is correlated with lymph node involvement in the surgical specimens. TRG therefore could have clinical implications in the future in order to provide tailored therapies.
T-downstaging, evidenced by TNM staging, is defined as the reduction of at least one T level measured initially by endorectal ultrasound and/or pelvic magnetic resonance imaging and finally by pathological evaluation. Pathologic complete response is defined as the absence of tumor cells in the surgical specimen.
This work describes the efficacy of chemoradiation therapy in local advanced rectal cancer and concludes that lymph node metastasis is associated with the treatment failure. The writing is good and the conclusion is considerable.
P- Reviewer: Albulescu R, Hsu CP
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBO-CAN 2008, cancer incidence and mortality world-wide: IARC CancerBase No.10 (Internet). 2010; Available from: http://globocan.iarc.fr. |
| 2. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] |
| 3. | Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [PubMed] |
| 5. | Drebber U, Madeja M, Odenthal M, Wedemeyer I, Mönig SP, Brabender J, Bollschweiler E, Hölscher AH, Schneider PM, Dienes HP. β-catenin and Her2/neu expression in rectal cancer: association with histomorphological response to neoadjuvant therapy and prognosis. Int J Colorectal Dis. 2011;26:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Jakob C, Liersch T, Meyer W, Baretton GB, Schwabe W, Häusler P, Kulle B, Becker H, Aust DE. Prognostic value of histologic tumor regression, thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer UICC Stage II/III after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2006;30:1169-1174. [PubMed] |
| 7. | Beddy D, Hyland JM, Winter DC, Lim C, White A, Moriarty M, Armstrong J, Fennelly D, Gibbons D, Sheahan K. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2008;15:3471-3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Rosenberg R, Nekarda H, Zimmermann F, Becker K, Lordick F, Hofler H, Molls M, Siewert JR. Histopathological response after preoperative radiochemotherapy in rectal carcinoma is associated with improved overall survival. J Surg Oncol. 2008;97:8-13. [PubMed] |
| 9. | Colombo PE, Patani N, Bibeau F, Assenat E, Bertrand MM, Senesse P, Rouanet P. Clinical impact of lymph node status in rectal cancer. Surg Oncol. 2011;20:e227-e233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Sobin LH, Wittekin Ch. International Union Against Cancer (UICC) TNM classification of malignant tumors, 6th edn. Willey-Liss, New York. 2002;. |
| 11. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 12. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 465] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 13. | Weiser MR, Fichera A, Schrag D, Boughey JC, You YN. Progress in the PROSPECT trial: precision treatment for rectal cancer. Bull Am Coll Surg. 2015;100:51-52. [PubMed] |
| 14. | Ptok H, Meyer F, Steinert R, Vieth M, Ridwelski K, Lippert H, Gastinger I. No prognostic impact of isolated lymphovascular invasion after radical resection of rectal cancer--results of a multicenter observational study. Int J Colorectal Dis. 2007;22:749-756. [PubMed] |
| 15. | Losi L, Luppi G, Gavioli M, Iachetta F, Bertolini F, D’Amico R, Jovic G, Bertoni F, Falchi AM, Conte PF. Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis. 2006;21:645-651. [PubMed] |
| 16. | Moreno García V, Cejas P, Blanco Codesido M, Feliu Batlle J, de Castro Carpeño J, Belda-Iniesta C, Barriuso J, Sánchez JJ, Larrauri J, González-Barón M. Prognostic value of carcinoembryonic antigen level in rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2009;24:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Suárez J, Vera R, Balén E, Gómez M, Arias F, Lera JM, Herrera J, Zazpe C. Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Colorectal Dis. 2008;10:563-568. [PubMed] |
| 18. | Tsujinaka S, Kawamura YJ, Konishi F, Aihara H, Maeda T, Mizokami K. Long-term efficacy of preoperative radiotherapy for locally advanced low rectal cancer. Int J Colorectal Dis. 2008;23:67-76. [PubMed] |
| 19. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [PubMed] |
| 20. | García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298-304. [PubMed] |
| 21. | Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U, Silva E Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90-99; discussion 99-101. [PubMed] |
| 22. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [PubMed] |
| 23. | Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 24. | Tytherleigh MG, Ng VV, Pittathankal AA, Wilson MJ, Farouk R. Preoperative staging of rectal cancer by magnetic resonance imaging remains an imprecise tool. ANZ J Surg. 2008;78:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Huh JW, Park YA, Jung EJ, Lee KY, Sohn SK. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J Am Coll Surg. 2008;207:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, Kim J, Garcia-Aguilar J. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013;56:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, Wagman R, Saltz LB, Wong WD. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-135; discussion 135-136;. [PubMed] |
| 28. | Mignanelli ED, de Campos-Lobato LF, Stocchi L, Lavery IC, Dietz DW. Downstaging after chemoradiotherapy for locally advanced rectal cancer: is there more (tumor) than meets the eye. Dis Colon Rectum. 2010;53:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Read TE, Andujar JE, Caushaj PF, Johnston DR, Dietz DW, Myerson RJ, Fleshman JW, Birnbaum EH, Mutch MG, Kodner IJ. Neoadjuvant therapy for rectal cancer: histologic response of the primary tumor predicts nodal status. Dis Colon Rectum. 2004;47:825-831. [PubMed] |
| 30. | Zmora O, Dasilva GM, Gurland B, Pfeffer R, Koller M, Nogueras JJ, Wexner SD. Does rectal wall tumor eradication with preoperative chemoradiation permit a change in the operative strategy. Dis Colon Rectum. 2004;47:1607-1612. [PubMed] |
| 31. | Park IJ, You YN, Skibber JM, Rodriguez-Bigas MA, Feig B, Nguyen S, Hu CY, Chang GJ. Comparative analysis of lymph node metastases in patients with ypT0-2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2013;56:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 32. | Berho M, Oviedo M, Stone E, Chen C, Nogueras J, Weiss E, Sands D, Wexner S. The correlation between tumour regression grade and lymph node status after chemoradiation in rectal cancer. Colorectal Dis. 2009;11:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Elezkurtaj S, Moser L, Budczies J, Müller AJ, Bläker H, Buhr HJ, Dietel M, Kruschewski M. Histopathological regression grading matches excellently with local and regional spread after neoadjuvant therapy of rectal cancer. Pathol Res Pract. 2013;209:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121-1130. [PubMed] |
| 35. | Dhadda AS, Dickinson P, Zaitoun AM, Gandhi N, Bessell EM. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer. Eur J Cancer. 2011;47:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441. [PubMed] |
| 37. | Beresford M, Glynne-Jones R, Richman P, Makris A, Mawdsley S, Stott D, Harrison M, Osborne M, Ashford R, Grainger J. The reliability of lymph-node staging in rectal cancer after preoperative chemoradiotherapy. Clin Oncol (R Coll Radiol). 2005;17:448-455. [PubMed] |
| 38. | Wichmann MW, Müller C, Meyer G, Strauss T, Hornung HM, Lau-Werner U, Angele MK, Schildberg FW. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137:206-210. [PubMed] |
| 39. | de Campos-Lobato LF, Stocchi L, de Sousa JB, Buta M, Lavery IC, Fazio VW, Dietz DW, Kalady MF. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think! Ann Surg Oncol. 2013;20:3398-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Marks JH, Valsdottir EB, Rather AA, Nweze IC, Newman DA, Chernick MR. Fewer than 12 lymph nodes can be expected in a surgical specimen after high-dose chemoradiation therapy for rectal cancer. Dis Colon Rectum. 2010;53:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Govindarajan A, Gönen M, Weiser MR, Shia J, Temple LK, Guillem JG, Paty PB, Nash GM. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Habr-Gama A, Perez RO, Proscurshim I, Rawet V, Pereira DD, Sousa AH, Kiss D, Cecconello I. Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: what does it mean. Dis Colon Rectum. 2008;51:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Sprenger T, Rothe H, Becker H, Beissbarth T, Homayounfar K, Gauss K, Kitz J, Wolff H, Scheel AH, Ghadimi M. Lymph node metastases in rectal cancer after preoperative radiochemotherapy: impact of intramesorectal distribution and residual micrometastatic involvement. Am J Surg Pathol. 2013;37:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Pettersson D, Lörinc E, Holm T, Iversen H, Cedermark B, Glimelius B, Martling A. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg. 2015;102:972-978; discussion 978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |