Published online Aug 27, 2015. doi: 10.4240/wjgs.v7.i8.133
Peer-review started: April 29, 2015
First decision: May 14, 2015
Revised: May 26, 2015
Accepted: June 30, 2015
Article in press: July 2, 2015
Published online: August 27, 2015
Processing time: 125 Days and 5.1 Hours
To describe the etiology, anatomy and pathophysiology of rectovaginal fistulas (RVFs); and to describe a systematic surgical approach to help achieve optimal outcomes. A current review of the literature was performed to identify the most up-to-date techniques and outcomes for repair of RVFs. RVFs present a difficult problem that is frustrating for patients and surgeons alike. Multiple trips to the operating room are generally needed to resolve the fistula, and the recurrence rate approaches 40% when considering all of the surgical options. At present, surgical options range from collagen plugs and endorectal advancement flaps to sphincter repairs or resection with colo-anal reconstruction. There are general principles that will allow the best chance for resolution of the fistula with the least morbidity to the patient. These principles include: resolving the sepsis, identifying the anatomy, starting with least invasive surgical options, and interposing healthy tissue for complex or recurrent fistulas.
Core tip: There are general principles that will allow the best chance for resolution of a rectovaginal fistula with the least morbidity to the patient. Identifying and addressing the disease process that caused the fistula is critical, including medical management for Crohn’s, and resolving inflammation or sepsis with a seton. Then the exact anatomy of the fistula should be defined to determine operative approaches. The operative algorithm should begin with fistula plugs and local advancement flaps, if these fail more invasive options such as diversion, and interposition of healthy tissue should be pursued for complex and recurrent fistulas.
- Citation: Kniery KR, Johnson EK, Steele SR. Operative considerations for rectovaginal fistulas. World J Gastrointest Surg 2015; 7(8): 133-137
- URL: https://www.wjgnet.com/1948-9366/full/v7/i8/133.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i8.133
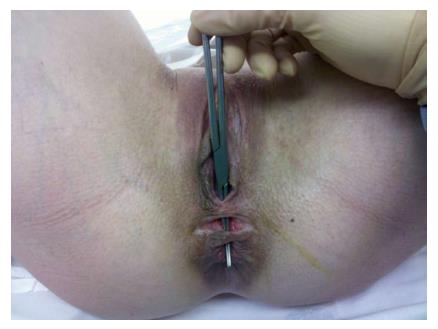
Rectovaginal fistula (RVF) is an epithelial lined tract between the rectum and vagina, and generally presents with passage of air, stool or even purulent discharge from the vagina (Figure 1). This can result in recurrent urinary tract or vaginal infections, but also creates a serious psychosocial burden for the patient[1]. They are well known to dramatically lower a female’s self-esteem and prevent successful intimate relationships. Unfortunately, they are also notoriously difficult to manage, despite the numerous surgical options presently described, and may even require fecal diversion to aid closure. When choosing the optimal method to surgically manage these fistulas, the available literature is limited and there currently are no large prospective trials comparing the numerous surgical options. While the paucity of data is driven in part by the relatively low incidence of RVFs and the complex anatomical differences between individual patients, it remains one of the more challenging conditions that surgeons caring for colorectal disease encounter. In this manuscript we will describe the scope and pathophysiology of RVFs, as well as a systematic approach to treating these patients and determining the most suitable operative approach.
RVFs account for approximately 5% of all perirectal fistulas, most commonly occurring as a result of obstetric trauma (85%) and pelvic surgery (5%-7%); while inflammatory bowel disease, malignancy, and radiation therapy encompass the majority of the remaining etiologies[1]. Although obstetric trauma causes the vast majority of RVFs, they are still relatively uncommon in this population, occurring in only approximately 0.1% of vaginal deliveries in Western countries[2]. In contrast, RVFs are considered almost endemic in sub-Saharan Africa and South Asia secondary to obstetrical trauma, with an estimated incidence of 50000 to 100000 new cases annually[2]. With a prevalence of two million, RVFs in developing nations are related to prolonged labors that cause necrosis of the rectovaginal septum. Overall, the past quarter century has seen the rates of episiotomy and operative vaginal delivery decrease dramatically, and with it the number of RVFs. Yet, vaginal deliveries associated with severe perineal lacerations, shoulder dystocia, operative vaginal delivery and prolonged and obstructed labor still occur and remain the highest risk for causing a RVF[3].
Outside of delivery complications, hysterectomy and rectal surgery are the highest risk procedures for causing RVFs. Use of stapling devices (specifically the double-stapled technique) and placement of perineal or vaginal mesh also have been shown to be associated with an increase in the likelihood of RVF formation[3]. The incidence of RVF after a resection for low rectal cancer is widely variable (0.9% to 10%), likely reflecting the heterogeneity in both the individual tumor and operating surgeon. Another possibility is that an anastomotic leak and the resulting pelvic sepsis may lead to the development of a RVF. To avoid the inciting event (i.e., leak), fecal diversion is commonly utilized following a proctectomy and low-lying anastomosis to “protect” it and minimize the clinical consequence of a leak. Although proximal diversion may play a role in improving outcomes (and is itself used in the management of RVFs), fecal diversion does not completely eliminate the risks of RVF, with up to 11% of patients after a proctocolectomy developing RVFs despite complete enteric diversion[2].
Another setting where RVFs can occur is in the setting of malignancy. Anal cancer, rectal cancer and pelvic cancer can all cause RVFs by various mechanisms. First, the lesion itself can be locally destructive, resulting in direct erosion between the two luminal surfaces. Another potential source of the RVF is from the adjuvant radiation therapy that is commonly used to help treat these pelvic malignancies. In this situation, the radiation is cytotoxic, leading to obliterative endarteritis, chronic inflammation and ischemia, and eventually resulting in a fistula between the two anatomical structures[2]. With regards to inflammatory bowel disease, RVFs are most commonly seen in Crohn’s disease and rarely in ulcerative colitis. While still relatively infrequent, women with Crohn’s disease have a reported cumulative 10% lifetime risk of developing a RVF. Of these, Crohn’s patients who have a significant disease burden in their colon are the most likely to be affected by RVFs[2]. While ulcerative colitis patients, especially following total proctocolectomy and ileal-anal pouch procedures, may still develop a RVF, this should be a “red flag” to providers to re-evaluate the patient for the possibility of a misdiagnosis of Crohn’s disease.
Although several classifications of RVFs exist, most RVF are generally broken down into low vs high fistulas and simple vs complex fistulas. These basic categorizations are extremely helpful in selecting the optimal surgical procedure for the patient. Low fistulas are generally located through or distal to the sphincter complex, but proximal to the dentate line. Due primarily to their location, they may be approached via anal, perineal or vaginal routes. Anovaginal fistulas have a rectal opening distal to the dentate line and are generally approached the same as a low fistula. High fistulas are proximal to the sphincteric complex, with a vaginal opening near the cervix, and generally require an abdominal approach for repair.
The other classification (simple vs complex) primarily differentiates the RVF on whether it will be amenable to a local repair vs a more complicated underlying pathogenesis that will require resection, interposition grafts, and/or diversion. A simple fistula is one that is smaller in size (< approximately 2.5 cm), more distally located along rectovaginal septum, and generally occurred a result of trauma or a cryptograndular infection. Complex fistulas are typically a result of inflammatory bowel disease, radiation or invasive cancer. Fistulas that have failed prior attempts at repair are also included in the category. Complex fistulas are commonly more proximal on the rectovaginal septum and are not amenable to primary repair, though may occur anywhere due to the underlying etiology.
To optimize outcomes, it is important to ensure that any associated perineal sepsis has resolved completely before attempting an operative repair. This should be achieved primarily by addressing the underlying cause of the fistula (e.g., medical therapy for Crohn’s disease, removal of a foreign body such as a staple, or drainage of an abscess). Once this has been addressed, adjunctive measures such as fecal diversion or a draining seton will help resolve the active inflammation and allow the tissues to soften and be more amenable to operative repair.
The anatomy of the individual patient and the fistula itself are the foremost factors in determining which procedure to perform. In general, our approach has been to recommend an attempt at less invasive procedures first, and if those fail, to then try more complex and potentially morbid procedures. However, depending on the underlying disease state of the patient, individual co-morbidities and the anatomy of the fistula, a more “complex” repair that includes diversion may be recommended at the initial operation (Table 1).
| Published number of cases | Success rate | Complications | Fistula anatomy | |
| Advancement flaps | 515[10,11] | 68% | Incontinence, Recurrence, Larger Fistula | Low |
| Transperineal/sphincteroplasty | 72[12,13] | 64%-100% | Incontinence, Sexual dysfunction, Wound Dehiscence | Low |
| Gracilis muscle flap | 99[14,15] | 43%-100% | Sexual dysfunction, Cosmesis, Wound dehiscence | Low + High |
| Plugs | 49 | 45.9% | Recurrence, Cost | Low |
| Transabdominal ligation1 | 49[16,17] | 95%-100% | Bleeding, Intraperitoneal Rectal injuries | High |
| Mesh repair | 48[10,18] | 71%-81% | Recurrence, Larger fistula, Cost | Low + High |
| Martius flap | 104[7,19] | 65%-100% | Sexual Function, Cosemsis | Low |
The plugs currently available are composed of synthetic material or made from porcine small intestine submucosa. Regardless of the composition, the tract is debrided, and the plug is brought through the RVF fistula in an attempt to form a biologic seal. In some cases, surgeons will perform a concomitant endorectal advancement flap with plug placement to improve outcomes. Fistula plugs have shown some benefit in perianal fistulas of cryptoglandular origin; yet, the limited data for RVFs has shown only a 20%-50% closure rate. The length of the tract, which is almost always very short, likely plays a role in the high failure rate of this procedure, as has been seen with anal fistulas having short tracts[4].
Advancement flaps may be performed by raising either rectal or vaginal mucosa and using it to cover the fistulous tract. This is performed in conjunction with debridement/excision of the fistula tract and primary closure. Healthy surrounding tissue is mobilized along a wide pedicle to ensure adequate blood supply and brought distally to cover the RVF. Different opinions exist as to the best approach. Those that favor an endorectal flap feel it is easier to mobilize and approximate the rectal mucosa when compared with vaginal mucosa, and that the repair is performed from the high-pressure side. Proponents of the vaginal side feel it is better vascularized, less likely to result in a larger fistula, and an easier recovery. In either instance, the reported success rates of this repair are reported between 60%-90%. In general, this is the procedure of choice for low-lying/simple traumatic RVFs without a history of incontinence[4].
A transperineal repair is accomplished by approaching the fistula tract through the perineum, making an incision at the perineal body and dissecting in the rectovaginal septum above the level of the fistula. The tract is then excised, and closure is performed in multiple layers on both the sides. The benefit of this approach is that an overlapping sphincteroplasty can be performed simultaneously for those patients that have associated defects or in those patients in which the fistula can be incorporated into the sphincter repair. This is best used in women with preexisting incontinence, or those a history of failed transanal or transvaginal approach[2]. Success rates are reported to be 64.7%-100%; however, this procedure is often more technically challenging, results in higher morbidity rates, and normally is not a first-line procedure[4].
In 1928 Dr. Heinrich Martius, a professor of gynecology in Gottingen, described using the bulbocavernosus muscle and labial fat pad for vaginal wall defects due to its proximity which allows for a single operative field[5]. The Martius flap was first used in cysto- and urethral-vaginal fistulas. Only later was it adapted to its present use in RVFs. In sum, it is ideally suited for RVF repair, providing a local well-vascularized pedicle of adipose/muscular tissue that is mobile and results in low morbidity. It is most suited for complex, recurrent, or recalcitrant RVFs[6]. The Martius flap is best able to treat low and mid-level fistulas up to approximately 5 cm proximal to the vaginal introitus, but in reality is only limited by the reach of the bulbocavernosus pedicle.
There are approximately 104 cases reported in the retrospective literature with a success rate ranging from 65%-100%[4]. Dyspareunia has been reported in as many as 30% of females at six weeks post operatively when they are allowed to resume vaginal intercourse, but it appears to improve with time. The only other more common complication reported in the literature are labial wound issues (< 10%), which largely resolve with local wound care[7].
In this procedure, the gracilis muscle is harvested from the leg, mobilized on a proximal pedicle, and used as an interposition graft between the rectum and vagina. Success rates are reported from 60%-100%, but there is increased morbidity associated with the harvest site and there appears to be a prolonged decrease in sexual function[4]. Dyspareunia is reported in up to 57% of patients undergoing this operation and the decreased sexual desire has been felt to be, in part, related to the relatively large burden of perineal scarring[8]. Furthermore, when the gracilis is harvested for use in other procedures (e.g., plastic surgery free flaps), a short-term decrease in functionality of that leg has been reported for approximately 6 mo in 26% of the patients, and 6% of patients have long-term difficulties[9].
Transabdominal ligation procedures are typically performed when the RVF is high (i.e., vaginal cuff), and may be performed via a minimally invasive or open approach. The common bond to these fistulas is often the presence of a prior hysterectomy and an inflammatory condition that resulted in pelvic sepsis that eroded through the vaginal cuff (e.g., Crohn’s diverticulitis, anastomotic leak). In this procedure, the offending bowel is resected along with division of the fistula tract. It is often helpful to place a piece of omentum in between the rectum and vagina to avoid recurrence. Some gynecologists prefer to debride and re-close the vaginal cuff, although this is widely variable. Success rates are 95%-100%, and normally this is the preferred treatment for the patient has a high fistula tract[4].
A mesh repair is essentially the same as transabdominal ligation. However, rather than placing omentum between the rectum and vagina, various biologic meshes have been utilized as an interposition graft between the two structures to prevent re-fistulization. The largest study used porcine small intestine submucosa and showed a success rate of 71%-81% in 48 patients. Other biologic meshes such as acellular porcine dermal graft and acellular human dermal matrix have also been successful in small studies and case reports[4]. Biological mesh placement has also been described following perineal approaches, although this is less well described.
RVFs are a disease process that is a significant burden on women that are afflicted, and a difficult problem for surgeons from whom they seek help. The diverse disease pathology has prevented prospective trials, and consensus guidelines on the management of these patients. With a clear understanding of the anatomy, ensuring resolution of the sepsis, and large armentarium of surgical approaches these patients can be treated successfully.
P- Reviewer: Coskun A, Wong KKY S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Ommer A, Herold A, Berg E, Fürst A, Schiedeck T, Sailer M. German S3-Guideline: rectovaginal fistula. Ger Med Sci. 2012;10:Doc15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (1)] |
| 2. | Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Brown HW, Wang L, Bunker CH, Lowder JL. Lower reproductive tract fistula repairs in inpatient US women, 1979-2006. Int Urogynecol J. 2012;23:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Göttgens KW, Smeets RR, Stassen LP, Beets G, Breukink SO. The disappointing quality of published studies on operative techniques for rectovaginal fistulas: a blueprint for a prospective multi-institutional study. Dis Colon Rectum. 2014;57:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (5)] |
| 5. | White AJ, Buchsbaum HJ, Blythe JG, Lifshitz S. Use of the bulbocavernosus muscle (Martius procedure) for repair of radiation-induced rectovaginal fistulas. Obstet Gynecol. 1982;60:114-118. [PubMed] |
| 6. | Kin C, Gurland B, Zutshi M, Hull T. Martius flap repair for complex rectovaginal fistula. Pol Przegl Chir. 2012;84:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 7. | McNevin MS, Lee PY, Bax TW. Martius flap: an adjunct for repair of complex, low rectovaginal fistula. Am J Surg. 2007;193:597-59; discussion 599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Lefèvre JH, Bretagnol F, Maggiori L, Alves A, Ferron M, Panis Y. Operative results and quality of life after gracilis muscle transposition for recurrent rectovaginal fistula. Dis Colon Rectum. 2009;52:1290-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Papadopoulos O, Konofaos P, Georgiou P, Chrisostomidis C, Tsantoulas Z, Karypidis D, Kostakis A. Gracilis myocutaneous flap: evaluation of potential risk factors and long-term donor-site morbidity. Microsurgery. 2011;31:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ellis CN. Outcomes after repair of rectovaginal fistulas using bioprosthetics. Dis Colon Rectum. 2008;51:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 11. | Lowry AC, Thorson AG, Rothenberger DA, Goldberg SM. Repair of simple rectovaginal fistulas. Influence of previous repairs. Dis Colon Rectum. 1988;31:676-678. [PubMed] |
| 12. | Wiskind AK, Thompson JD. Transverse transperineal repair of rectovaginal fistulas in the lower vagina. Am J Obstet Gynecol. 1992;167:694-699. [PubMed] |
| 13. | Athanasiadis S, Köhler A, Weyand G, Nafe M, Kuprian A, Oladeinde I. [Endo-anal and transperineal continence preserving closure techniques in surgical treatment of Crohn fistulas. A prospective long-term study of 186 patients]. Chirurg. 1996;67:59-71. [PubMed] |
| 14. | Wexner SD, Ruiz DE, Genua J, Nogueras JJ, Weiss EG, Zmora O. Gracilis muscle interposition for the treatment of rectourethral, rectovaginal, and pouch-vaginal fistulas: results in 53 patients. Ann Surg. 2008;248:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Fürst A, Schmidbauer C, Swol-Ben J, Iesalnieks I, Schwandner O, Agha A. Gracilis transposition for repair of recurrent anovaginal and rectovaginal fistulas in Crohn’s disease. Int J Colorectal Dis. 2008;23:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | van der Hagen SJ, Soeters PB, Baeten CG, van Gemert WG. Laparoscopic fistula excision and omentoplasty for high rectovaginal fistulas: a prospective study of 40 patients. Int J Colorectal Dis. 2011;26:1463-1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Schloericke E, Hoffmann M, Zimmermann M, Kraus M, Bouchard R, Roblick UJ, Hildebrand P, Nolde J, Bruch HP, Limmer S. Transperineal omentum flap for the anatomic reconstruction of the rectovaginal space in the therapy of rectovaginal fistulas. Colorectal Dis. 2012;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Schwandner O, Fuerst A, Kunstreich K, Scherer R. Innovative technique for the closure of rectovaginal fistula using Surgisis mesh. Tech Coloproctol. 2009;13:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Pitel S, Lefevre JH, Parc Y, Chafai N, Shields C, Tiret E. Martius advancement flap for low rectovaginal fistula: short- and long-term results. Colorectal Dis. 2011;13:e112-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |