Published online Apr 27, 2015. doi: 10.4240/wjgs.v7.i4.52
Peer-review started: June 24, 2014
First decision: August 17, 2014
Revised: February 11, 2015
Accepted: March 5, 2015
Article in press: March 9, 2015
Published online: April 27, 2015
Processing time: 293 Days and 13 Hours
AIM: To outline the feasibility, safety, adverse events and early results of endoscopic ultrasound (EUS)-radiofrequency ablation (RFA) in pancreatic neoplasms using a novel probe.
METHODS: This is a multi-center, pilot safety feasibility study. The intervention described was radiofrequency ablation (RF) which was applied with an innovative monopolar RF probe (1.2 mm Habib EUS-RFA catheter) placed through a 19 or 22 gauge fine needle aspiration (FNA) needle once FNA was performed in patients with a tumor in the head of the pancreas. The Habib™ EUS-RFA is a 1 Fr wire (0.33 mm, 0.013”) with a working length of 190 cm, which can be inserted through the biopsy channel of an echoendoscope. RF power is applied to the electrode at the end of the wire to coagulate tissue in the liver and pancreas.
RESULTS: Eight patients [median age of 65 (range 27-82) years; 7 female and 1 male] were recruited in a prospective multicenter trial. Six had a pancreatic cystic neoplasm (four a mucinous cyst, one had intraductal papillary mucinous neoplasm and one a microcystic adenoma) and two had a neuroendocrine tumors (NET) in the head of pancreas. The mean size of the cystic neoplasm and NET were 36.5 mm (SD ± 17.9 mm) and 27.5 mm (SD ± 17.7 mm) respectively. The EUS-RFA was successfully completed in all cases. Among the 6 patients with a cystic neoplasm, post procedure imaging in 3-6 mo showed complete resolution of the cysts in 2 cases, whilst in three more there was a 48.4% reduction [mean pre RF 38.8 mm (SD ± 21.7 mm) vs mean post RF 20 mm (SD ± 17.1 mm)] in size. In regards to the NET patients, there was a change in vascularity and central necrosis after EUS-RFA. No major complications were observed within 48 h of the procedure. Two patients had mild abdominal pain that resolved within 3 d.
CONCLUSION: EUS-RFA of pancreatic neoplasms with a novel monopolar RF probe was well tolerated in all cases. Our preliminary data suggest that the procedure is straightforward and safe. The response ranged from complete resolution to a 50% reduction in size.
Core tip: This manuscript presents a pilot, safety feasibility study with the results of the first in humans endoscopic ultrasound (EUS) guided radiofrequency ablation (RFA) for cystic neoplasms and neuroendocrine tumors of the pancreas with a novel EUS-RFA catheter. EUS-RFA is feasible and well tolerated. EUS-RFA with this novel catheter provides endoscopic treatment option other than surgical resection for pancreatic lesions.
- Citation: Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg 2015; 7(4): 52-59
- URL: https://www.wjgnet.com/1948-9366/full/v7/i4/52.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i4.52
Incidental pancreatic solid or cystic lesions are diagnosed with increased frequency due to the widespread use of abdominal cross-sectional imaging to investigate unrelated symptoms. In a large single-centre study, pancreatic cysts were diagnosed in 1.2% of 24000 individuals subjected to abdominal cross-sectional imaging[1]. As a result, the majority of these lesions are diagnosed at an earlier stage, before they become invasive and present with jaundice, pancreatitis or abdominal pain[2]. Lesions such as neuroendocrine tumors (NET), mucinous cystadenomas and intraductal papillary mucinous neoplasms have the potential of malignant transformation. This risk is lower with NET, but significantly higher with mucinous lesions[3].
The standard treatment of solid or cystic pancreatic lesions with malignant potential has been surgical resection, with lesions in the pancreatic head requiring a Whipple resection whereas pancreatic tail lesions are treated with distal pancreatectomy. Both types of resection carry significant morbidity and mortality, resulting in unacceptably high risk/benefit ratios for many elderly patients with co-morbidities[4,5]. Currently, patients deemed unfit for major pancreatic surgery are offered cross-sectional imaging surveillance at regular intervals according to the International Association of Pancreatology Guidelines[6]; these guidelines recommend annual imaging for lesions < 10 mm, 6-monthly imaging for cysts 10-20 mm and 3-monthly imaging for lesions larger than 20 mm. However, controversy exists regarding the optimal follow up of patients with primary pancreatic lesions, underlying the need for minimally invasive ablative techniques as alternative to surgical resection.
Radiofrequency ablation (RFA) has been used percutaneously and intraoperatively to treat primary and secondary liver cancers by achieving localized tumor necrosis[7-10]. Endo-biliary application of radiofrequency (RF) has been developed in our unit and used in patients with inoperable bile duct and pancreatic head adenocarcinomas presenting with biliary obstruction[11]. Many alternative techniques of endoscopic ultrasound (EUS)-guided tumor ablation have been described, including RF ablation, photodynamic therapy, laser ablation, and ethanol injection[12].
EUS-RFA could achieve complete ablation of pancreatic cysts with malignant potential in patients unfit for surgery, thus eliminating the requirement for long-term surveillance in this group of individuals. Gaidhane et al[13] showed that EUS-RFA of the pancreatic head using Habib EUS-RFA catheter (Emcision Ltd., United Kingdom) through a 19 gauge needle was well tolerated in 5 Yucatan pigs with minimum amount of pancreatitis. The aim of this study is to outline the safety, feasibility, adverse events and early results of EUS-RFA in patients with pancreatic neoplasms using a novel probe.
Eight patients were subjected to EUS-RFA of a neoplastic lesion in the head of the pancreas. A novel monopolar RF catheter [Habib™ EUS-RFA catheter, Emcision Ltd., London (CE Marked)] (Figure 1) was placed through a 19 or 22 gauge fine needle aspiration (FNA) needle.
Inclusion criteria were age over 18 years, patients with a cystic pancreatic lesions that were not suitable surgical candidates and patients that consented to participate in the study. Exclusion criteria included patients younger than 18 years, patients not consenting to participate in the study, uncorrected coagulopathy and cardiac pacemakers in situ.
All patients were investigated with blood tests; haematological, biochemical, tumor markers as well as radiological investigation including computed tomography scan and ultrasound scans. On follow-up, patients had clinical examination, blood tests and cross sectional imaging to assess the pancreatic lesion. The follow-up ranged from 3 to 6 mo. Data are presented as mean plus or minus standard deviations of the mean or median with range. Research was carried out in accordance with the Helsinki Declaration.
The Habib™ EUS-RFA is a 1 Fr wire (0.33 mm, 0.013”) with a working length of 190 cm, which can be inserted through the biopsy channel of an echoendoscope. RF power is applied to the electrode at the end of the wire to coagulate tissue in the liver and pancreas. This is a monopolar device and is used in conjunction with a patient grounding/diathermy pad.
Habib™ EUS-RFA catheter comes in a dispensing sheath. The catheter is removed from the dispensing sheath and connected to the adaptor cable, which is then connected to the generator. Power in the generator is set to the required wattage we used 5-25 Watts in our patient group). A patient grounding/diathermy pad is applied as close to the operating field as possible, since the catheter is monopolar. We applied the pad on the lower back of the patient. The entire area of the grounding pad should be reliably applied to the patient’s body to avoid skin burns.
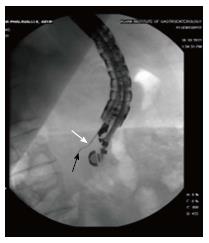
The echoendoscope is manoeuvred to obtain proper sonographic visualization of the target lesion. Under EUS control, a 19 gauge biopsy needle (with stylet) is introduced into the target lesion. In pancreatic cystic lesions, effort was made to completely aspirate the cyst before applying RFA. The tip of the needle was positioned near the far end of the lesion. In case of pancreatic NET also, the FNA needle was positioned at the deepest part of the tumor. The stylet is removed from the biopsy needle and Habib™ EUS RFA catheter is gently pushed inside the hollow of the biopsy needle until it cannot be pushed any further. Carefully maintaining this position of the Habib™ EUS RFA probe, the FNA needle is gradually withdrawn by 3 cm in order to disengage contact between the active part of the RF catheter located at the tip and the metallic FNA needle. Fluoroscopy assists in visualization of the RFA probe protruding beyond the tip of the needle (Figure 2). The tip of the probe is floppy, and may take a curved shape in emptied cystic lesons.
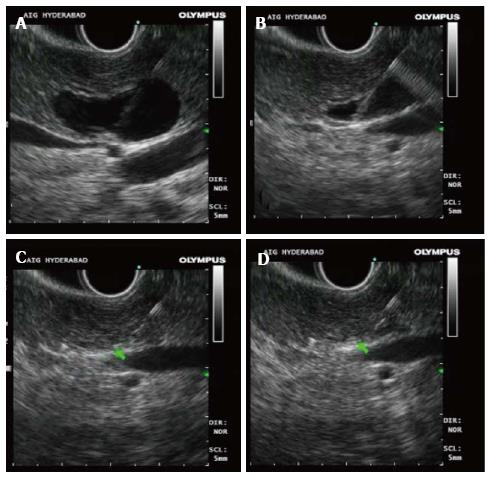
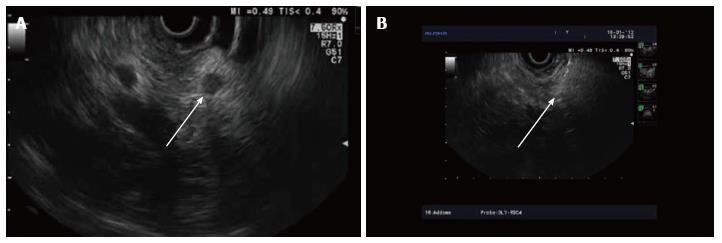
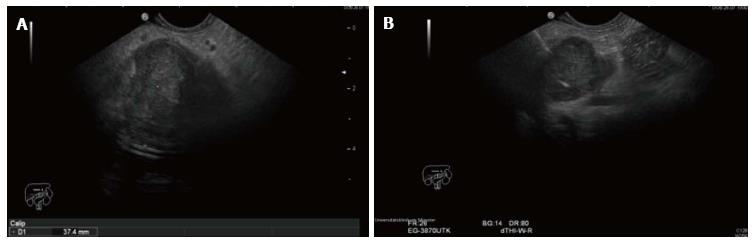
RF energy is applied for 90-120 s at the set wattage. In larger lesions, the Habib™ EUS RFA probe and needle is pulled back as one unit and repositioned to ablate near end of the lesion (Figures 3-5). This process can be repeated as many times, as needed to ensure complete ablation of the lesion. In larger pancreatic lesions, repeat puncture with the FNA needle is done in a different axis (after withdrawing the RFA probe, with or without replacing with stylet). The patients were managed post procedure as per standard hospital practice for EUS interventional procedures.
Eight patients [median age of 65 (range 27-82) years; 7 female and 1 male] were recruited in a prospective multicentre trial. Six had a pancreatic cystic neoplasm (four a mucinous cyst, one had IPMN and one a microcystic adenoma). In all six cases, diagnosis was based on imaging reviewed by an expert radiologist. The remaining two cases, had a NET in the head of pancreas (previously documented with diagnostic FNA cytology and not suitable for surgical intervention). The mean size of the cystic neoplasms and NETs were 36.5 mm (SD ± 17.9 mm) and 27.5 mm (SD ± 17.7 mm) respectively. RF [Rita (Model 1500X) or ERBE (Model ICC 200) was applied at 5 watts, 15 watts, 20 watts and finally 25 watts in 3, 2, 2 and one patients respectively over 90 s for each watt setting (Table 1). The median number of applications was 4.5 (range 2-7). Patients with cystic neoplasm and one patient with NET had one session of RFA each, whilst a second patient with NET had two sessions of RFA.
| Age | Sex | Diagnosis | No. of RF applications/session | No of sessions | Dead/alive |
| 5 Watts | |||||
| 82 | F | Mucinous cyst | 3 | 1 | Alive |
| 73 | F | Mucinous cyst | 5 | 1 | Alive |
| 46 | F | Microcystic adenoma | 5 | 1 | Alive |
| 15 Watts | |||||
| 40 | F | Mucinous cyst | 3 | 1 | Alive |
| 27 | F | Mucinous cyst | 2 | 1 | Alive |
| 20 Watts | |||||
| 57 | F | NET | 6 | 1 | Alive |
| 82 | F | NET | 4 | 2 | Alive |
| 25 Watts | |||||
| 78 | M | IPMN | 7 | 1 | Alive |
The EUS-RFA was completed in all cases. Amongst the 6 patients with pancreatic cystic neoplasm, the post procedure imaging in 3-6 mo showed complete resolution of the cysts in 2 patients, whilst in 3 patients there was 48.4% reduction [mean pre RF 38.8 mm (SD ± 21.7 mm) vs mean post RF 20 mm (SD ± 17.1 mm)] in size (Table 2). Using cross sectional imaging in 2 patients with NET, a change in vascularity and central necrosis after EUS-RFA was demonstrated. There were no episodes of post-procedural pancreatitis, perforation or bleeding within 48 h. Two patients had mild abdominal pain that resolved in 3 d.
| No. | Diagnosis | Pre ablationsize (mm) | Post ablation size (mm) | Adverse events |
| 1 | Mucinous cyst | 30 | 10 | No |
| 2 | Mucinous cyst | 40 | Cyst not seen | No |
| 3 | Microcystic adenoma | 20 | 8 | No |
| 4 | Mucinous cyst | 70 | 45 | Mild pain |
| 5 | Mucinous cyst | 24 | Cyst not seen | Mild pain |
| 6 | IPMN | 35 | 17 | No |
| 7 | NET | 15 | Change in vascularity | No |
| 8 | NET | 40 | Central area of necrosis 15 mm | No |
RFA is a well-recognized, safe and effective modality for the treatment of focal malignant diseases[14,15]. RFA uses high-frequency alternating current to generate thermal energy and thus coagulative necrosis to the tissue[16]. The technique is minimally invasive and has very good tolerability which are the major advantages[17]. RFA is increasingly applied in pancreatic lesions[18], including unresectable pancreatic carcinoma where RFA has an acceptable mortality but high morbidity[16,17,19-21]. In general, adverse events are associated with the duration of ablation. Pancreas is very thermo-sensitive, and when heat is applied on normal pancreas it produces an inflammatory response causing edema and later fibrosis and occasionally cystic transformation[18]. Massive necrosis of the pancreas following RFA have been reported, probably due to sequential ablations done in close proximity at the same session[17,20].
In recent years there have been reports of prospective studies using RFA in locally advanced pancreatic adenocarcinoma. In 2010, Girelli et al[22] reported ultrasound-guided RFA during laparotomy in fifty patients with locally advanced pancreatic cancer. In this prospective study the main outcome measures were short-term morbidity and mortality. In thirty four patients the tumor was located in the pancreatic head or the uncinate process and in 16 in the body or tail; median diameter was 40 (inter-quartile range 30-50) mm. Abdominal adverse events occurred in 24% of patients. Half of those were directly associated with RFA (two pancreatic fistulas and four cases of portal vein thrombosis) and were managed conservatively. When the applied heat was reduced from 105 degrees C to 90 degrees C there was a significant reduction in adverse events (ten vs two of 25 patients; P = 0.028). Median postoperative hospital stay was 10 (range 7-31) d. The authors concluded that RFA of locally advanced pancreatic cancer is feasible and relatively well tolerated. In another observational study, the same group compared patients with locally advanced pancreatic carcinoma treated with either primary RFA (group 1) or RFA following any other primary treatment (group 2)[23]. In total, 107 consecutive patients were treated with RFA of which 47 patients in group 1 and 60 in group 2. Median overall survival was 25.6 mo and it was significantly shorter in group 1 than in group 2 (14.7 mo vs 25.6 mo; P = 0.004). In this study the authors reported that RFA after alternative primary treatment was associated with prolonged survival.
RFA has been proposed by many groups as a strong adjuvant for antitumor response as it induces an immune response targeting tumor antigens[24-26]. In situ tumor destruction by RFA provides the immune system with an antigen for the induction of antitumor immunity. Antigen-presenting cells take up antigens in the periphery after which they induce specific immune responses[25]. Wissniowski et al[24] reported that RFA can induce a tumor-specific T-cell reaction in the non-reactive neoplasm-bearing host, probably by overcoming immune tolerance and leading to the presentation of otherwise cryptic neoplastic antigens. In another study, ablation of hepatocellular carcinoma (HCC) was found to induce a functional transient activation of myeloid dendritic cells associated with increased serum levels of TNF-α and IL-1β with a sustained antitumoral immune response[26]. Moreover, animals treated with subtotal RF ablation showed significant increases in tumor-specific class I and II responses to male minor histocompatibility (HY) antigens and tumor regression[27]. Subtotal RF ablation produces an enhanced systemic antitumor immune response and tumor regression which is related to increased dendritic cell infiltration. RFA can also induce a tumor-specific proliferative T cell response and even transplantable protective immunity[28].
Intraoperative RFA uses a larger device with higher energy and is associated with significant morbidity and mortality. However, EUS guided RFA is a more conservative approach and avoids surgical intervention. Goldberg et al[29] applied EUS guided RFA to the pancreas of 13 Yorkshire pigs at 285 ± 120 mA for 6 min resulting in discrete zones of coagulation necrosis in the porcine pancreas. Only one of the 13 animals had increased lipase levels and mild focal pancreatitis. No other significant adverse events were observed. A more recent study in 2008 demonstrated the feasibility and efficacy of EUS RFA using a newly developed bipolar ablation probe combining RFA and cryotechnology in 14 pigs. The size of the ablation achieved was related to the duration of ablation; when applied for 900 s there was a high complication rate in the healthy pancreas. Adverse events were less common compared to conventional RFA needles[18]. In a recent study by Kim et al[30], EUS-RFA of the pancreas was applied on 10 adult mini pigs. An 18 gauge endoscopic RFA probe was used to ablate the body and tail of the pancreas, with an output power of 50 W for 5 min. On histology, there was a spherical necrotic lesion surrounded by fibrous tissue localized in the pancreatic parenchyma. The mean diameter of the ablated tissue was 23.0 ± 6.9 mm. No major procedure-related adverse events were observed, and all pigs survived without any distressed behavioural pattern for 7 d until autopsy. Another minimally invasive technique for treatment of pancreatic cystic lesions with moderate success is the EUS-guided injection of ethanol into the cyst, with reported efficacy of 33.5%-62% in achieving cyst resolution[31,32]. The adverse events associated with this technique are significant, with a reported risk of severe post-procedural pain and pancreatitis of 4%-20%. Also, the presence of multiple septations within the cyst reduces the efficacy of ethanol injection. Another limitation of ethanol ablation is that this method would not be suitable for treatment of solid pancreatic lesions. A major potential advantage of EUS-RFA of cystic tumors is that it could be done in a minimally invasive way, with the likelihood of fewer adverse events than the alcohol injection because the area of ablation can be assessed and monitored in real-time by EUS.
EUS-RFA using Habib EUS-RFA catheter (Emcision Ltd., United Kingdom) through a 19 gauge needle for ablation of lymphatic and pancreatic tissue, was reported in two animal studies. In the former study[33], EUS-guided RFA ablation of mediastinal lymph nodes was successfully attempted in six pigs. RFA was performed with the ERBE Vaio generator (ERBE, Tuttlingen, Germany) with bipolar settings of 10 watts, effect 2 for 2 min. During the procedure, the probe was visible in all cases. No evidence of ablation effect in the surrounding tissue or at the needle puncture site was seen on gross examination. There was a direct correlation between the probe length and the size of necrosis. In the pancreatic study using the same catheter, five Yucatan pigs underwent EUS-guided RFA of the head of the pancreas[13]. RFA was applied with 6 mm of the probe exposed at 4 watts for 5 min, 5 watts for 0.9 min, and 6 watts for 0.2 min. Then, with 10 mm of the probe exposed in the pancreas, RFA was performed at 4 watts for 4.3 min, 5 watts for 1.4 min, and 6 watts for 0.8 min. Autopsy showed moderate level of pancreatitis, with involvement of 20% of the proximal pancreatic tissue in only one pig. There was minimal tissue damage in the other animals. In this study EUS-guided RFA of the pancreatic head with the monopolar probe through a 19 gauge needle was well tolerated with a minimal amount of pancreatitis.
We have reported in this prospective study the application of RFA via the novel Habib EUS-RFA catheter (Emcision Ltd., United Kingdom) for pancreatic cystic neoplasms and NET. The concept of treating pre-malignant asymptomatic pancreatic lesions by means other than surgical resection is appealing, as the latter is associated with major morbidity and some mortality. This study shows that such an approach is feasible and safe. Our patients were discharged hours without any major adverse events. However, it is conceivable that the application of RF energy in the pancreatic parenchyma may be associated with some adverse events. Such adverse events may include (but not necessarily limited to) acute pancreatitis, pancreatic leaks, infection of necrotic pancreatic tissue post treatment and bleeding. Using lower energy also allows for repeating the ablation with low morbidity as per clinical indication. EUS-RFA of pancreatic neoplasms with a novel monopolar RF probe was well tolerated in all patients. These preliminary data results suggest that the procedure is technically easy and safe. The response ranged from complete resolution to a 50% reduction in diameter. Further multicenter experience is required before widespread use of this novel procedure.
Nagy Habib is shareholder and director of EMcision which designed and developed the device. None of the other authors have a conflict of interest or a financial disclosure to declare.
The aim of this report is to outline the feasibility, safety, adverse events and early results of endoscopic ultrasound guided radiofrequency ablation (EUS-RFA) in pancreatic neoplasms using a novel RF probe. The HabibTM EUS-RFA is monopolar catheter with a 1 Fr wire (0.33 mm, 0.013”) with a working length of 190 cm, which can be inserted through the biopsy channel of an echoendoscope. RF power is applied to the electrode at the end of the wire to coagulate tissue in the liver and pancreas.
This first in human study shows that EUS-RFA with this novel catheter provides an endoscopic treatment option other than surgical resection for pancreatic lesions.
The standard treatment of solid or cystic pancreatic lesions with malignant potential has been surgical resection. Pancreatic resections carry significant morbidity and mortality, resulting in unacceptably high risk/benefit ratios for many elderly patients with co-morbidities. There is an unmet need for minimally invasive ablative techniques as alternative to surgical resection.
Our results show that the procedure is technically easy and safe. The response in this series ranged from complete resolution to a 50% reduction in diameter. Therefore it might be an excellent alternative for patients that are not suitable surgical candidates.
Radiofrequency ablation is the procedure of destructing tissue with the use of heat generated from high frequency alternating current (in the range of 350-500 kHz). It is a widely accepted method of tissue destruction for primary solid organ tumors. It has been used in the management of primary liver and lung tumors in patients that are not suitable surgical candidates and in secondary malignancies as part of the treatment algorithm.
This author congratulated demonstrating wonderful study.
P- Reviewer: Cidon EU, Chiu KW, Ogura T, Tepes B, Watanabe M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651-657; discussion 657-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Kitagawa Y, Unger TA, Taylor S, Kozarek RA, Traverso LW. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12-18; discussion 18-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (34)] |
| 5. | Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1441] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 7. | Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999;177:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Pai M, Frampton AE, Mikhail S, Resende V, Kornasiewicz O, Spalding DR, Jiao LR, Habib NA. Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol. 2012;38:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Pai M, Spalding D, Jiao L, Habib N. Use of bipolar radiofrequency in parenchymal transection of the liver, pancreas and kidney. Dig Surg. 2012;29:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 12. | Yoon WJ, Brugge WR. Endoscopic ultrasonography-guided tumor ablation. Gastrointest Endosc Clin N Am. 2012;22:359-369, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Gaidhane M, Smith I, Ellen K, Gatesman J, Habib N, Foley P, Moskaluk C, Kahaleh M. Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of the Pancreas in a Porcine Model. Gastroenterol Res Pract. 2012;2012:431451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Chen MH, Yang W, Yan K, Gao W, Dai Y, Wang YB, Zhang XP, Yin SS. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005;11:6395-6401. [PubMed] |
| 15. | Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, Takagi H, Mori M, Nakajima T. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006;12:608-611. [PubMed] |
| 16. | Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Campagnol M, Ambrosi A, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Siriwardena AK. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP. 2006;7:1-4. [PubMed] |
| 20. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2007;96:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Cantore M, Girelli R, Mambrini A, Frigerio I, Boz G, Salvia R, Giardino A, Orlandi M, Auriemma A, Bassi C. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg. 2012;99:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Wissniowski TT, Hänsler J, Neureiter D, Frieser M, Schaber S, Esslinger B, Voll R, Strobel D, Hahn EG, Schuppan D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496-6500. [PubMed] |
| 25. | den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 332] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, Bocher WO, Endrulat K, Blum HE, Geissler M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Kim HJ, Seo DW, Hassanuddin A, Kim SH, Chae HJ, Jang JW, Park do H, Lee SS, Lee SK, Kim MH. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Sethi A, Ellrichmann M, Dhar S, Hadeler K-GERD, Kahle E, Seehusen F, Klapper W, Habib N, Fritscher-Ravens A. 503 EUS-Guided Lymph Node Ablation With Novel Radiofrequency Ablation Probe: A Feasibility Study. Gastrointest Endosc. 2012;75:AB147. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |