Published online Mar 27, 2015. doi: 10.4240/wjgs.v7.i3.33
Peer-review started: September 12, 2014
First decision: November 27, 2014
Revised: January 8, 2015
Accepted: January 18, 2015
Article in press: January 20, 2015
Published online: March 27, 2015
Processing time: 201 Days and 13.2 Hours
AIM: To retrospectively evaluate the long-term survival of patients that received radiofrequency ablation (RFA) therapies of colorectal liver metastases.
METHODS: In 2005 to 2008, RFA of 105 colorectal liver metastases (CRLM) were performed on 49 patients in our institution. The liver metastases were evaluated, both before and after ablation therapies, with contrast enhanced computerised tomography and contrast enhanced ultrasonography. Histological evidence of malignant liver metastases was obtained in the few instances where contrast enhanced ultrasonography gave equivocal results. Accesses to the CRLM were guided ultrasonically in all patients. The data obtained from records of these ablations were retrospectively analysed and survival data were compared with existing studies in the literature.
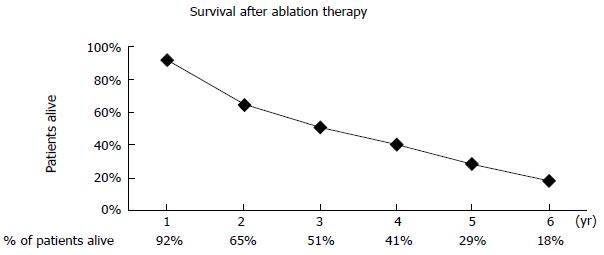
RESULTS: 1-, 2-, 3-, 4- and 5-year survival rates, when no stringent selection criteria were applied, were 92%, 65%, 51%, 41% and 29% respectively. To explore the impact of the number and size of CRLM on patients’ survival, an exclusion of 13 patients (26.5%) with number of CRLM ≥ 5 and tumour size ≥ 40 mm resulted in 1-, 2-, 3-, 4- and 5-year survival rates improving to 94%, 69%, 53%, 42% and 31% respectively. It is of note that 9 of 49 patients developed extra-hepatic metastases, not visible or seen on pre-treatment scans, just after RFA treatment. These patients had poorer survival. The development of extra-hepatic metastases in nearly 20% of the patients included in our study can partly account for modestly lower survival rates as compared with earlier studies in the literature.
CONCLUSION: Our study underscores the fact that optimum patients’ selection before embarking on RFA treatment is vitally important to achieving a superior outcome.
Core tip: The current study corroborates the consensus in the literature which proposes that adequate patients’ selection before radiofrequency ablation (RFA) therapy is vitally important to achieving a satisfactory ablation success. To the best of our knowledge, the consensus proposed that patients with more than 5 hepatic metastases and tumour size of more than 40 mm are probably unsuitable for RFA. Furthermore, inadvertent inclusion of patients with extra-hepatic metastases for RFA treatment of colorectal liver metastases is an important factor that can influence negatively the overall patients’ survival.
- Citation: Babawale SN, Jensen TM, Frøkjær JB. Long-term survival following radiofrequency ablation of colorectal liver metastases: A retrospective study. World J Gastrointest Surg 2015; 7(3): 33-38
- URL: https://www.wjgnet.com/1948-9366/full/v7/i3/33.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i3.33
Cancers constitute a great economic burden in the western world. Colorectal cancer is the third most common cancer across the world and is ranked the second most frequent cause of cancer associated mortality in the industrialised countries[1,2]. Around 50% of colorectal cancer patients will eventually develop liver metastases[2-5]. So, effective control of colorectal liver metastases (CRLM) has the potential of improving patients’ survival.
The traditional mode of treating CRLM has been surgically with quoted 5 year survival rate varying from 24% to 58% in carefully selected patients[3,5-7]. Surgical resection has some significant inherent drawbacks; mortality can be as high as 10% peri-operatively and up to 37% of patients undergoing hepatic resection could end up with profound morbidity[3]. Resection of several hepatic metastases has the potential of leaving behind a significant low hepatic reserve. Resection of metastatic lesions in the liver close to vital structures such as major vessels cannot be safely carried out[3].
Radiofrequency ablation (RFA) as one of the techniques to achieving local control of CRLM has received intense attention in recent years. Development and incessant improvement of RFA techniques as a treatment modality of CRLM aim to reaching similar patients’ survival as in surgical treatment with fewer complications[3]. The main goal of this study was to retrospectively evaluate the long-term survival of patients that received ablation therapies of CRLM in our institution compared with earlier studies in the literature with the intention to ascertain optimum quality control of our applied RFA technique.
Wealth of data for this study originated from the electronic patient chart database, picture archiving and communication system (PACS) and records of RFA therapy. From 2005 to 2008, ablations of liver metastases were performed at the Aalborg University Hospital on 49 patients (32 men and 17 women) who had colorectal cancer. The demography of the patients is presented in Table 1 and is essentially similar to other studies in the literature[6,8,9]. It was not possible, at the time of diagnosis, to establish in all patients whether the liver metastases were synchronous or metachronous with the primary tumour. Twenty patients (40.8%) received at least one additional session of RFA therapy due to either local tumour recurrence or development of new liver metastases.
| Total number of patients (n): 49 | No. of women: 17 |
| Mean age: 65 yr | No. of men: 32 |
| No. of patients | |
| Age distribution | |
| ≤ 50 yr | 3 (6) |
| 51-79 yr | 44 (90) |
| ≥ 80 yr | 2 (4) |
| Total number of liver metastases ablated: 105 | |
| Average numbers of metastases per patient: 2.3 | |
| No. of liver metastases ablated | |
| ≤ 3 | 40 (82) |
| 4-7 | 8 (16) |
| ≥ 8 | 1 (2) |
| Maximum size of metastases ablated | |
| Size of metastases | |
| < 10 mm | 3 (6.1) |
| 11-30 mm | 31 (63.3) |
| 31-39 mm | 6 (12.2) |
| ≥ 40 mm | 9 (18.4) |
Because the institution was only modestly experienced in the ablation technique in 2005-2008, strict and well defined inclusion criteria were not clearly outlined before embarking on CRLM ablation therapy. The pre-RFA scans were evaluated according to the best clinical practice, and none of the included patients had any clear signs of extra-hepatic metastases. However, we saw some non-specific lung nodules in the pre-RFA scans of some patients, where some of these nodules later turned out to be metastases. Patients were accepted for ablation therapy irrespective of numbers and sizes of the liver metastases. All patients had resection of their colorectal primary tumour and received chemotherapy in oncologic regime.
A grand total of 105 liver metastases were primarily ablated (Table 1). A significant proportion of patients (82%) had ≤ 3 liver metastases, 8 patients had 4-7 liver metastases and only one patient had 8 liver metastases at the time of diagnosis. The largest size of a single ablated lesion was 70 mm.
The diagnoses of colon tumour and hepatic metastases were established with the aid of contrast enhanced computerised tomography (CECT). In most cases, hepatic metastases were also confirmed by contrast enhanced ultrasonography (CEUS) to aid the planning of RFA procedures. Histological evidence of malignant liver metastases was obtained in the few instances where CEUS gave equivocal results. Two consultant radiologists with several years of experience evaluated CECT and CEUS in all patients.
The same protocols as for pre-treatment diagnostic imaging evaluation were repeated for follow-up post treatment. Post treatment imaging assessments were carried out at 1 mo and thereafter 3 monthly post-ablation treatments if there were no evidences of recurrence or new metastases.
We defined primary ablation success in terms of lack of abnormal hepatic contrast enhancement (in CECT and CEUS) at 1 mo post treatment imaging. Enhancement at the border of earlier site of ablation was termed local tumour recurrence (LTR). Newly discovered abnormally enhancing lesions in follow-up imaging that were neither clear in the pre-treatment scanning nor related to earlier ablation sites, were dubbed new hepatic metastases (NHM). Presence of LTR or NHM or both qualified patients for additional session(s) of RFA.
RFA were guided ultrasonically in all patients. Vast majority of liver ablations were carried out percutaneously. In few cases where liver metastases could not be reached safely percutaneously or because of limited visualization, RFA were carried out under ultrasound guidance following laparotomy. All patients had ablations under local and general anaesthesia. During percutaneous ablations, patients were positioned appropriately to ensure the best visualization of target lesions in the liver. In few cases, CEUS were utilized to increase the confidence of tumour visibility under ablation therapy.
The size of each metastasis to be ablated dictated the choice of RFA electrode. In a small sized tumour (< 3 cm), single internally cooled electrode (Cool-tipTM Ablation Electrodes, ACT2530, Covidien, CO, United States) was utilized. In a large sized tumour (≥ 3 cm), either a single electrode with repeated overlapping ablations or cluster electrode with 3 electrodes contained in a single applicator (Cool-tipTM Ablation Electrodes, ACT2015, Covidien, CO, United States) was used. Each electrode was powered by the attached generator (Cool-tipTM Ablation Generator E series, Covidien, CO, United States) and tissue temperature around the tip of the electrode placed appropriately in the tumour was continuously monitored. Each electrode in the target tissue was powered continuously for 12 min and average final tissue temperature reached was 65 °C.
The manuscript was supervised by a co-author, Jens Brøndum Frøkjær, with extensive statistical expertise.
A total of 105 liver metastases were ablated. Twenty-eight piont six percent of patients (14 of 49) received ablation therapies at a time frame less than one month after the detection of CRLM. Thirty-eight piont eight percent (19 of 49) and thirty-two piont seven percent (16 of 49) respectively had treatment 1-3 mo and > 3 mo after diagnoses of liver metastases.
Primary ablation success was achieved in 95.2% (100 of 105) of CRLM at first month post-ablation treatment. To put in another way, only 4.8% of ablated tumours had local recurrence at 1 mo following ablation therapy. However, 15 new liver metastases were diagnosed within one month after liver ablation treatment. The range of survival from dates of diagnosing CRLM was 10 to 93 mo (median survival of 28.5 mo and mean survival of 35.5 mo). Only 18.4% (9 of 49) of patients survived beyond 93 mo. 1-, 2-, 3-, 4- and 5-year survival rates were respectively 92%, 65%, 51%, 41% and 29% (Figure 1).
To explore the impact of number and size of CRLM on patients’ survival, we re-analysed our data based on introduction of certain hypothetical exclusion criteria; exclusion of patients with number of CRLM ≥ 5 and tumour size ≥ 40 mm. This is in accordance with the recently introduced recommendation[10-14]. Following the introduction of these criteria, thirteen patients (26.5%) were excluded, resulting in the improvement of 1-, 2-, 3-, 4- and 5-year survival rates as depicted in Table 2.
| Years | 1 | 2 | 3 | 4 | 5 |
| % of patients alive | 94 | 69 | 53 | 42 | 31 |
Sub-analysis showed that 9 of 49 patients developed extra-hepatic metastases, not visible on pre-treatment scans, just after RFA treatment. These patients had poorer survival.
Colorectal cancer (CRC) is ranked the third commonest malignancy in the world[1,2]. Large proportions of patients with CRC are susceptible to developing liver metastases[15,16]. Uncontrolled secondary malignant liver lesions, including CRLM, are among the major sources of mortality and morbidity in patients diagnosed with CRC[10]. CRLM is invariably fatal if left untreated[17,18]. The dismal quoted median survival of untreated CRLM is 12 mo[17].
To improve patients’ survival, a number of treatment modalities have been developed. Surgical resection is widely acknowledged as the gold standard of treating secondary liver malignancy[6,15,17,19,20]. It is argued that surgical resection can effectively cure liver metastases[5,21] and that local recurrence rate is low as well as increased chance of long disease-free interval[15,18]. Improvements in patients’ assessment and surgical techniques have been suggested as the factors that improved the patients’ survival following surgical excision of CRLM in recent years[22]. However, more than 70% of patients with CRLM are not suitable candidates for surgery at the time of diagnosis due to diverse factors such as co-morbidity, unfavourable tumour stage, limited liver reserve and proximity of liver lesions to vital structures[3,5,7,21]. So, a different modality of treatment had to be advanced.
Some local treatments of CRLM that have been tried include RFA, microwave ablation, cryotherapy and percutaneous ethanol injection[8,23,24]. RFA is widely accepted as a promising alternative to achieving local control of CRLM because of associated fewer complications[16,25-27]. In other words, mortality and morbidity are comparably insignificant and efficacy of tumour ablation in patients treated with RFA is impressive[8,21,24]. RFA is also deemed to be a safe and effective procedure[11,12,19,28]. Despite the numerous benefits of RFA, it is not without some shortcomings. One of the undesirable entities that could negatively impact success rate following liver ablation therapy is local tumour recurrence. The factors that have been attributed to hepatic tumour recurrence following RFA are large sizes and multiplicity of CRLM. It is advocated that the number of CRLM ablated per patient should be at most 5[9,11,14]. Some studies also proposed that patients with more than 4 to 5 metastatic liver lesions are probably unsuitable for RFA therapy[10,26]. The ideal size of CRLM to be targeted for ablation is still a subject of much discussion. Some suggested that the largest size of CRLM to be ablated should be ≤ 30 mm[4,10,11,21] while others were of the opinion that the largest size should be ≤ 40 mm[13,14,28]. The inferential consensus from the above statements is that the maximum numbers of CRLM per patient should be ≤ 5 and each with size of ≤ 40 mm to achieve a high ablation success[4,10,12-14]. It is immediately clear that strict patients’ selection largely determines the degree of success in RFA treatment of CRLM.
In our study, 1-year survival rate of 92% is favourably comparable to other three selected studies in the literature (Table 3). Our 5-year survival rate of 29%, without applying strict exclusion criteria, is apparently on the lower side as compared with other RFA therapy studies. This impression would definitely appear less gloomy if the comparison is made with the 5-year survival rate (ranging from 24% to 58%) in patients treated mainly with surgical resection[3,5,6]. Adam et al[29] reported even lower 5-year survival rate of 18% when survival was estimated in connection with resection of CRLM in patients who had extra-hepatic metastases. Considering the nature of our studies in which patients were not strictly selected, our estimated 5-year survival rate might not be absolutely unsatisfactory. In most of the earlier studies, patients were meticulously selected. As noted earlier, we did not apply stringent criteria to patients’ selection in 2005. Eighteen percent of our patients had at least four CRLM and a similar proportion had an individual tumour size of at least 40 mm. It should be noted that 2% of our patients had eight CRLM. It is reasonably obvious that the results of our patients’ overall survivals would have been modestly better if we applied the generally agreed principle that at most 5 tumours[9,11,14] and individual tumour size ≤ 40 mm[4,11-13,28] be considered for RFA therapy to substantially minimize the risk of local tumour recurrence and thereby improving patients’ survival. This is partly supported by a modest improvement in our results following the application of hypothetical strict exclusion criteria (Table 2).
It is of note that 9 of 49 patients in our study developed extra-hepatic metastases, not visible or seen on pre-treatment scans, just after RFA treatment. These patients had the worst overall survivals and this can partly explain why the overall survivals of our patients were modestly lower. It is immediately clear that optimum patients’ selections resulting from initial careful patients’ assessment and meticulous pre-RFA evaluation have profound influence on patients’ survival. It is probable that paying limited attention to strict patients’ selection could account for some of the disappointing results seen at other institutions introducing RFA technique.
To moderate the tumour recurrence rate in connection with RFA, technique has to be continuously improved. As modestly experienced as our institution was in 2005-2008, our RFA technique was quite effective. A staggering 95.2% primary ablation success rate was accomplished at first month post-ablation treatment. This figure is comparable to the one (93.1%) reported by Solbiati et al[6].
The potential downside of our study is the difficulty we encountered in providing convincing data to establish a guideline for optimum patients’ selection before embarking on RFA treatment of CRLM. The major reason for this probable shortcoming is the small number of patients included in the study. Forty-nine (49) patients that underwent RFA of CRLM between the years 2005 and 2008 were preliminary included in the study to allow for 5 year follow-up and estimation of preliminary survival rates. Other patients that received similar treatment after 2008 are being followed closely and data originating from this are being collated for future large study and publication. Besides, we chose to have our preliminary data published to excite interest in further research with the possibility of establishing widely acceptable optimum guidelines for performing minimally invasive treatment of CRLM. The success emanating from such research, in no doubt, will have a positive impact on myriads of patients across the world diagnosed with CRLM.
Number and sizes of CRLM as well as the presence of extra-hepatic metastases are among the most important factors that influence the outcomes of patients treated with RFA. Even though our study did not convincingly establish precise inclusion criteria, it underscored the fact that optimum patients’ selection before embarking on RFA treatment is critically important to achieving a superior outcome. We are of the opinion that further research is necessary to outline widely accepted criteria for selecting patients for RFA therapy.
Colorectal cancer is a common cancer worldwide and ranks second among the cancers that frequently cause deaths globally. A significant proportion of patients diagnosed with colorectal cancer are prone to developing the spread of the disease to the liver. The spread of the disease to the liver is one of the reasons for the prevalence of high mortality and morbidity associated with colorectal cancer. Effective controls of the cancer at the primary site (colorectum) as well as the metastatic spread to the liver are extremely important in improving patients’ survival.
To improve patients’ survival, a number of treatment modalities have been extensively explored. Surgical resection is widely acknowledged as the gold standard of treating metastatic liver disease. In addition, other treatment modalities that have been developed include radiofrequency ablation, microwave ablation, cryotherapy and percutaneous ethanol injection.
In recent years, attention has been shifted away from treatment of metastatic liver cancers with surgical resection due to the inherent risks associated with surgery and the fact that more than 70% of patients were unsuitable for surgery at the time of diagnosing metastatic colorectal cancer. Radiofrequency ablation, a procedure that is deemed safe and effective, is a widely accepted alternative for controlling metastatic liver disease because of associated fewer complications compared with surgical resection.
One of the undesirable entities that could negatively impact the success rate following liver radiofrequency ablation therapy is local tumour recurrence. In order to prevent local tumour recurrence and thereby improving patients’ survival, guideline for optimum patients’ evaluation and selection pre-treatment should be established. In our study, the authors explored the impacts of various factors that have been attributed to tumour recurrence following radiofrequency ablation of metastatic liver cancer. The published data will, in no doubt, excite interest in further research with the possibility of establishing widely acceptable optimum guidelines for performing minimally invasive treatment of metastatic liver cancer. The success emanating from such research will have a positive impact on myriads of patients across the world diagnosed with metastatic colorectal cancer.
Metastasis is the spread of cancerous cells from a primary site of origin to other parts of the body. Metastatic colorectal liver cancer is therefore, the spread of cancerous cells from the colorectum to the liver. Radiofrequency ablation of metastatic liver cancer is a minimally invasive treatment modality in which metastatic tumours in the liver are thermally destroyed by introducing high frequency electric current from a generator to the tumour via the electrode(s) inserted through the skin, under imaging guidance, to the tumour in the liver.
The manuscript is well written.
P- Reviewer: Bandyopadhyay SK, Bramhall S, Tandon R S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | McKay A, Dixon E, Taylor M. Current role of radiofrequency ablation for the treatment of colorectal liver metastases. Br J Surg. 2006;93:1192-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Bhardwaj N, Strickland AD, Ahmad F, Dennison AR, Lloyd DM. Liver ablation techniques: a review. Surg Endosc. 2010;24:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Ardito F, Vellone M, Cassano A, De Rose AM, Pozzo C, Coppola A, Federico B, Giovannini I, Barone C, Nuzzo G. Chance of cure following liver resection for initially unresectable colorectal metastases: analysis of actual 5-year survival. J Gastrointest Surg. 2013;17:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Vogl TJ, Müller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. Liver metastases: interventional therapeutic techniques and results, state of the art. Eur Radiol. 1999;9:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Sørensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol. 2007;48:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Vogl TJ, Straub R, Eichler K, Söllner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;230:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Sofocleous CT, Sideras P, Petre EN. “How we do it” - a practical approach to hepatic metastases ablation techniques. Tech Vasc Interv Radiol. 2013;16:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Buscarini E, Savoia A, Brambilla G, Menozzi F, Reduzzi L, Strobel D, Hänsler J, Buscarini L, Gaiti L, Zambelli A. Radiofrequency thermal ablation of liver tumors. Eur Radiol. 2005;15:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132:605-611; discussion 611-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Elias D, Baton O, Sideris L, Matsuhisa T, Pocard M, Lasser P. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol. 2004;11:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Bale R, Widmann G, Schullian P, Haidu M, Pall G, Klaus A, Weiss H, Biebl M, Margreiter R. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol. 2012;22:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, Poston G. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis. 2011;13:e252-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Sindram D, Lau KN, Martinie JB, Iannitti DA. Hepatic tumor ablation. Surg Clin North Am. 2010;90:863-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20:S342-S347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Ayav A, Germain A, Marchal F, Tierris I, Laurent V, Bazin C, Yuan Y, Robert L, Brunaud L, Bresler L. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg. 2010;200:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Gravante G, Overton J, Sorge R, Bhardwaj N, Metcalfe MS, Lloyd DM, Dennison AR. Radiofrequency ablation versus resection for liver tumours: an evidence-based approach to retrospective comparative studies. J Gastrointest Surg. 2011;15:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Mayo SC, Pawlik TM. Thermal ablative therapies for secondary hepatic malignancies. Cancer J. 2010;16:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Ng KK, Poon RT. Radiofrequency ablation for malignant liver tumor. Surg Oncol. 2005;14:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: Actual limitations and potential solutions in the future. World J Hepatol. 2011;3:8-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Taibbi A, Furlan A, Sandonato L, Bova V, Galia M, Marin D, Cabibbo G, Soresi M, Bartolotta TV, Midiri M. Imaging findings of liver resection using a bipolar radiofrequency electrosurgical device--initial observations. Eur J Radiol. 2012;81:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kosari K, Gomes M, Hunter D, Hess DJ, Greeno E, Sielaff TD. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg. 2002;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 475] [Article Influence: 19.8] [Reference Citation Analysis (0)] |