Published online Apr 27, 2014. doi: 10.4240/wjgs.v6.i4.77
Revised: December 2, 2013
Accepted: February 18, 2014
Published online: April 27, 2014
Processing time: 217 Days and 23.1 Hours
Neuroendocrine carcinoma (NEC) is a rare tumor, comprising < 1% of stomach cancers. A 55-year-old woman was referred to our hospital with biopsy-proven gastric cancer. A shallow ulcerative lesion was detected in the lesser curvature of the lower body. It was suspected to be early gastric cancer IIA + IIC type. Thus, endoscopic submucosal dissection was performed. She was subsequently diagnosed with NEC, which is aggressive and carries a poor prognosis. We conducted a radical resection and a laparoscopic-assisted distal gastrectomy. The tumor had infiltrated the subserosal layer and 6/42 lymph nodes were involved. The mitotic index was 16/10 high power fields and the Ki-67 labeling index was 26%-50%. The final diagnosis of NEC was made according to the World Health Organization 2010 criteria. She was suspected of having jumping metastasis to the proximal margin. The patient was treated with an oral anticancer drug (5-flurouracil based drug) for 2 years. The patient has been followed up for 3 years without recurrence.
Core tip: Some studies argue that neuroendocrine carcinoma (NEC) can be removed by endoscopic resection. However, in this case, we found that NEC can have jumping metastasis. Thus, NEC must be removed by radical surgical resection.
- Citation: Kang SH, Kim KH, Seo SH, An MS, Ha TK, Park HK, Bae KB, Choi CS, Oh SH, Choi YK. Neuroendocrine carcinoma of the stomach: A case report. World J Gastrointest Surg 2014; 6(4): 77-79
- URL: https://www.wjgnet.com/1948-9366/full/v6/i4/77.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i4.77
Neuroendocrine carcinoma (NEC) is rare tumor that includes < 1% of stomach cancers. It is aggressive and has a poor prognosis[1-3]. NEC is classified as neuroendocrine carcinoma G3 according to The World Health Organization (WHO) classification of tumors of the digestive system, 2010[4].
In this report, we describe a patient with NEC who underwent endoscopic submucosal dissection (ESD) and laparoscopic assisted distal gastrectomy (LADG) for removal of a tumor.
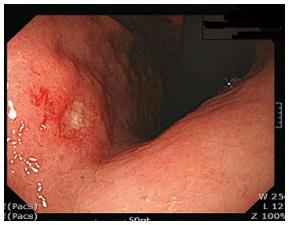
A 55-year-old woman with acid reflux underwent an esophagogastroduodenoscopy (EGD) for a checkup. A shallow ulcerative lesion was detected in the lesser curvature of the lower body (Figure 1). It was suspected to be early gastric cancer IIA + IIC type. A biopsy was done and it was diagnosed as a well differentiated adenocarcinoma. She was transferred to the digestive department of our hospital.
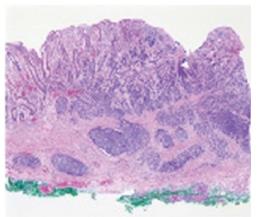
On July 13, she underwent ESD. The specimen was 6.3 cm × 3.8 cm and a pathological examination revealed a 1.2 cm × 1.4 cm NEC that had invaded the submucosal layer. The tumor cells exhibited mitosis in 16/10 high power fields (HPF). The resection margin was clear (Figure 2) and no lymphatic, vascular or neural invasion was observed. She was advised to undergo an operation due to possible neural invasion by the NEC. On July 24, she vomited blood from an ulcer because of the weakened mucosa after ESD. The bleeding was stopped under emergency EGD. She underwent conservative treatment with a proton-pump inhibitor and no oral intake.
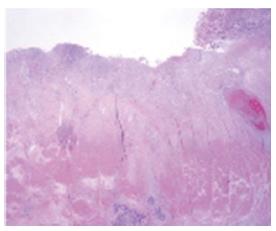
On August 9, she underwent LADG with a D2 lymphadenectomy. A Billroth type I anastomosis was done. A frozen biopsy revealed that the proximal and distal resection margins were clear of lesions. The mass was 2.2 cm × 1.3 cm in size and limited to the subserosa (Figure 3). The proximal resection margin was very close to the lesion but the distal resection margin was clear. Neural and lymphatic invasion was observed with 6 of 42 metastatic lymph nodes harvested. The tumor cells were positive for synaptophysin, chromogranin and CD56. The Ki-67 labeling index was 2+ (26%-50%). These findings led to the diagnosis of NEC, according to the 2010 WHO criteria[4]. The proximal margin was clear but the final pathology showed that some cancer cells were found between the mucosa and submucosa.
Minor bleeding was detected through the drain during the first 3 d. After an antihemorrhagic treatment and a transfusion, the blood tests were stable and the drain color changed to clear. She was discharged after the drain was removed.
She was treated with an oral anticancer drug [5-flurouracil (5-FU) based drug] for 2 years. No recurrence at the anastomosis or other site in the stomach was observed 3 years later.
Neuroendocrine neoplasm (NEN) is an epithelial neoplasm with predominant neuroendocrine differentiation and is an uncommon tumor with multiple sites of occurrence[5].
NENs are commonly divided by origin as located in the foregut (lung, bronchus, stomach or duodenum), midgut (jejunum, ileum, appendix or proximal colon) and hindgut (distal colon or rectum). The percentage of foregut cases is 34%, midgut 30% and hindgut 36%[6].
Gastric NEN is classified into neuroendocrine tumor (NET), neuroendocrine carcinoma (NEC), mixed adenoneuroendocrine carcinoma, enterochromaffin cells, serotonin-producing NETs and gastrin-producing NETs. NETs include NET G1 (carcinoid) and NET G2 (well-differentiated neuroendocrine tumor/carcinoma). NECs include NEC G3 (poorly differentiated neuroendocrine carcinoma small cell type/large cell type)[4]. NEN is positive for synaptophysin and chromogranin A[7].
NEN is classified based on the level of cellular proliferation, including the mitotic and Ki-67 indices[4]. In our case, the mitotic index was 16/10 HPF and the Ki-67 labeling index was 26%-50%. Thus, she was diagnosed with NEC. We suspected jumping metastasis from the main lesion to the proximal margin.
Gastric NEN has different prognoses and treatments depending on type. The prognosis of NET G1 is good and the 5 year survival rate is high. NET G2 has a favorable prognosis but is aggressive. NEC has the highest malignant potential but the 5 year survival rate is 75%-80%; however, the prognosis is poor. NET can be removed by endoscopic resection, whereas NEC requires surgical resection and lymph node dissection[8]. The best choice adjuvant chemotherapy for NEC is cisplatinum-based chemotherapy[9]. However, in this case we used a 5-FU oral agent because of the patient’s financial status and compliance.
In conclusion, a neuroendocrine tumor can be removed by endoscopic resection but it must be a radical surgical resection in accordance with a malignant tumor, due to its aggressive tendency and high malignant potential.
This case reports a neuroendocrine carcinoma with jumping metastasis.
Neuroendocrine neoplasm can be diagnosed using a mitotic count and the Ki-67 index.
Neuroendocrine tumors can be removed by endoscopic resection but a neuroendocrine carcinoma must be excised by radical surgical resection.
In this case, the authors used 5-flurouracil chemotherapy, but a common choice for neuroendocrine carcinoma is cisplatinum-based chemotherapy.
P- Reviewers: Voutsadakis IA, Walenkamp AME S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 279] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Rindi G. Clinicopathologic aspects of gastric neuroendocrine tumors. Am J Surg Pathol. 1995;19 Suppl 1:S20-S29. [PubMed] |
| 3. | Yu JY, Wang LP, Meng YH, Hu M, Wang JL, Bordi C. Classification of gastric neuroendocrine tumors and its clinicopathologic significance. World J Gastroenterol. 1998;4:158-161. [PubMed] |
| 4. | The International Agency for Research on Cancer. WHO Classification of Tumors of the Digestive System. 4th edition. Bosman FT, Carneiro F, Hruban RH, Theise ND, editor. Lyon: International Agency of Research of Cancer 2010; 53-57. |
| 5. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1181] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 6. | Klöppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2005;19:507-517. [PubMed] |
| 7. | Vyberg M, Horn T, Francis D, Askaa J. Immunohistochemical identification of neuron-specific enolase, synaptophysin, chromogranin and endocrine granule constituent in neuroendocrine tumours. Acta Histochem Suppl. 1990;38:179-181. [PubMed] |
| 8. | Kim BS, Oh ST, Yook JH, Kim KC, Kim MG, Jeong JW, Kim BS. Typical carcinoids and neuroendocrine carcinomas of the stomach: differing clinical courses and prognoses. Am J Surg. 2010;200:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Okita NT, Kato K, Takahari D, Hirashima Y, Nakajima TE, Matsubara J, Hamaguchi T, Yamada Y, Shimada Y, Taniguchi H. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |