Published online Apr 27, 2014. doi: 10.4240/wjgs.v6.i4.65
Revised: January 15, 2014
Accepted: March 17, 2014
Published online: April 27, 2014
Processing time: 174 Days and 4.5 Hours
Intrahepatic cholangiocarcinomas (ICC) are malignant tumors arising from the intrahepatic bile ducts that frequently recur after resection. The main sites of recurrence are the remnant liver, lymph nodes and lungs. Metastasis to the pancreas has never been reported. This case describes a 24-year-old woman who underwent a hepatic lobectomy in 2008 for an ICC. Almost 4 years after her surgery she presented with a pancreatic mass and lung nodules. An endoscopic ultrasound guided fine needle aspiration of the pancreatic mass and a video-assisted thoracoscopic surgery resection for the lung nodules were performed for diagnostic purposes. Pathological analyses of specimens revealed recurrence of her primary ICC in both pancreas and lungs. Subsequently, the patient received systemic chemotherapy. The patient is currently off chemotherapy and remains well. Moreover, she is pregnant. This is the first report of an ICC with pancreatic metastasis.
Core tip: Intrahepatic cholangiocarcinoma (ICC) is characterized by its high potential to metastasize. Most frequent sites for metastases are the remnant liver, lymph nodes and lungs. Metastasis to the pancreas has never been described. Although this may happen exceedingly rarely, hepatobiliary surgeons should be made aware that ICC can also metastasize to the pancreas.
- Citation: Labgaa I, Carrasco-Avino G, Fiel MI, Schwartz ME. Pancreatic recurrence of intrahepatic cholangiocarcinoma: Case report and review of the literature. World J Gastrointest Surg 2014; 6(4): 65-69
- URL: https://www.wjgnet.com/1948-9366/full/v6/i4/65.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i4.65
Cholangiocarcinomas are malignant tumors arising from the biliary tree. They account for about 3% of all digestive cancers and are the second most common primary liver tumors following hepatocellular carcinoma. In the United States approximately 5000 new cases are diagnosed each year[1] but the frequency considerably varies worldwide[2,3]. There are well-established risk factors as well as controversial ones. The former include primary sclerosing cholangitis, parasitic infections and biliary anomalies whereas the latter include inflammatory bowel diseases, obesity, diabetes, smoking and liver inflammatory conditions such as cirrhosis, hepatitis C and hepatitis B (HBV)[2-4]. Cholangiocarcinomas are divided into three different types according to their anatomic location along the biliary tree: intrahepatic cholangiocarcinomas (ICC), perihilar or Klatskin tumor (PCC) and distal extrahepatic cholangiocarcinoma[5]. Tumor features and behavior seem to vary according to its type, thus, the importance of a precise classification that will influence the management and eventual outcomes. ICC are located above the second-order bile duct that represents the segregation point from PCC. They account for approximately 10%-20% of all primary liver cancers)[2-4] and their incidence has been reported to increase disturbingly, especially within Western countries[6-8]. It is also characterized by its poor prognosis despite liver resection although surgery is considered as the only curative treatment. Studies have reported a 3-year survival rate of 22%-55% after extended surgery[9-13] whereas survival rate without surgical treatment was much poorer at 7%-21%[8,9,12]. The reason for this could be that ICC are longer clinically silent being often diagnosed at an advanced stage but also their strong tendency to recur. Postoperative recurrences were mainly located in the remnant liver whereas extrahepatic recurrences especially involved lymph nodes, lungs and peritoneum[9,14]. To our knowledge there is no case of pancreatic metastasis from ICC being reported in the literature. Thus, this case report is the first to address this interesting issue.
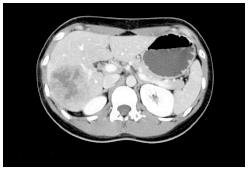
In May 2008 a healthy 24-year-old Chinese woman long-time immigrant was referred to our Division for the investigation of a liver mass revealed by an ultrasound at an outside hospital, as part of her regular follow up for chronic hepatitis B. A computed tomography (CT) scan with nonionic contrast confirmed a mass within segments VI-VII measuring 7.2 cm × 6.0 cm. No lesion was observed in the lungs and her pancreas appeared normal (Figure 1). The patient had no health issue beside HBV, received no medication and had not undergone any surgery so far. Her brother also had HBV but her family history for liver cancer was negative. She presented without symptoms and was not icteric. Abdominal palpation was unremarkable with a negative Murphy’s sign. Laboratory tests were performed and reported normal white cell count and hemoglobin. Kidney function and liver function were unremarkable. Tumors markers AFP and CA 19-9 were normal, 1.9 ng/mL and 8.4 U/mL, respectively. Based on the imaging studies, the pre-operative diagnosis was hepatocellular carcinoma. The patient underwent a right hepatic lobectomy and cholecystectomy. At surgery, the uninvolved liver appeared normal and there was no evidence of extrahepatic disease in the lymph nodes or anywhere else in the abdomen.
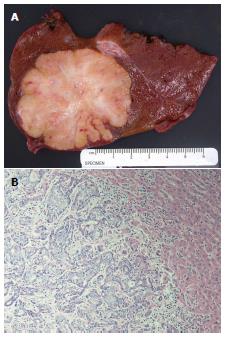
Pathology: A right liver lobe resection specimen was received and revealed a 6 cm × 5.5 cm × 5 cm white tan well-circumscribed firm mass with scalloped borders (Figure 2A). The tumor was 2 cm from the closest resection margin. Microscopically, the tumor consisted of moderately-differentiated intrahepatic cholangiocarcinoma characterized by anastomosing cords and glands with marked cytological atypical and embedded in dense stroma (Figure 2B). No lymphovascular invasion was noted. The bile duct margin was negative; no lymph nodes were identified from the hilar soft tissue that was entirely submitted. Carcinoma-in-situ and dysplastic changes involving adjacent bile ducts were seen. The uninvolved liver showed portal fibrosis but no portal inflammation. Rare ground-glass hepatocytes were idenfied. Immunohistochemical stain for hepatitis B surface antigen showed scattered hepatocytes with positive cytoplasmic staining, whereas hepatitis B core antigen was negative, findings that confirm hepatitis B infection.
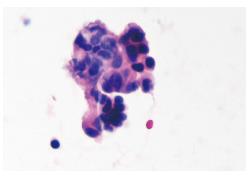
The patient recovered with no complication and was discharged 5 d after surgery. She was then routinely followed-up with CT of the chest and abdomen, tumor markers and complete lab tests on an outpatient mode. In January 2012, approximately 2 mo after delivering her baby and almost 4 years after her prior surgery, a CT-scan of the abdomen performed in an outside hospital highlighted a pancreatic ductal dilatation, suspicious to be secondary to a mass in the tail of the pancreas and nine nodules on both sides of the chest; each lesion was then confirmed by a positron emission tomography-computed tomography. In order to determine the nature of the pancreatic lesion, an endoscopic ultrasound (EUS)-Fine needle aspiration (FNA) was performed. In order to clarify the nature of the lung nodules the patient underwent a left video-assisted thoracoscopic surgery wedge resection. The specimen revealed a white firm well-circumscribed lesion measuring 1 cm × 1 cm with free margins. The findings supported metastatic cholangiocarcinoma (Figure 3). Surgery having no role in systemic cholangiocarcinoma, our plan was to introduce chemotherapy with Gemcitabine/Cisplatin for 3 mo followed by restaging.
Currently, the patient is off chemotherapy and remains very well. She is pregnant G2P1, due to have a baby in March 2014.
Intrahepatic cholangiocarcinomas are malignant neoplasms arising from the biliary tree, beyond the second order[5]. They represent approximately 10%-20% of all primary liver cancers[2-4]. ICC include different growth types: mass-forming, periductal-infiltrating and intraductal-growth[5]. Furthermore, they display a very malignant potential leading to a high risk of recurrence and a poor prognosis. Surgery, via liver transplant and hepatic resection, is considered as the only curative treatment for ICC[15]. Notwithstanding long-term outcomes are still far from reaching the expectancy. Most patients with ICC present recurrence within 2 years after surgery[9,10,12,14]. Not surprisingly survival rates are low. Despite an aggressive approach Konstadoulakis et al[13] reported 1-year, 3-year and 5-year survival rates of 80%, 49% and 25%, respectively[13]. Many potential predictor factors have been suggested. Concerning the well-established ones, several studies demonstrated the negative impact of positive margins[8,12,13,16-18]. Lymph nodes metastasis has also been identified as negative predictive factor although the benefit of lymphadenectomy is still debatable[8,10,11,14,16,17]. Intrahepatic cholangiocarcinomas have the potential to invade Glissonean sheath[16] leading to hematogenous, lymphatic or direct extension, causing dissemination of the disease. The absence of other lesion or peri-pancreatic adenopathy supports the hypothesis of hematogenous spreading although the dissemination pattern remains unclear in this case.
Concerning the liver lesion, as above mentioned, “carcinoma-in-situ and dysplastic changes involving adjacent bile ducts were seen”. This finding supports the diagnosis of primary cholangiocarcinoma, rather than metastatic tumor. This finding, associated with cytological features: (high N/C ratios, pleomorphism and high mitotic rates), permit to confidently rule-out the differential diagnosis of cholangiolocellular carcinoma[19,20].
Considering the pancreatic lesion, its morphology has been compared with the hepatic one; they were considered similar. Unfortunately, no tissue from the pancreatic FNA specimen is available for immunohistochemical studies. If tissue was available we may have add breast cancer markers to rule out metastatic breast cancer that could be considered in a young female patient.
The metastatic lesion in the pancreas could be explained as hematogenous spread from lesions in the lungs.
In term of risk factors the patient was HBV carrier. The role of HBV in ICC needs to be clarified. Although several studies considered it as a risk factor[2-4], a recent study suggested HBV could be a favorable prognostic factor after resection[21]. Liver fluke infestation was not tested. The patient did not present any other major risk factor but her recent history of pregnancy should be addressed although its role remains uncertain. Little is known in this field but clinical courses of ICC worsened by gravid state have been reported[22,23]. Indeed the high concentration of estrogen and the suppression of the immune system arising from pregnancy could potentially promote recurrence of ICC like it can aggravate preexisting liver lesion[24]. Chemotherapy is considered as the standard care for extrahepatic recurrences while surgery is not the gold standard in these cases[15]. Nevertheless data are strongly limited in this field and further studies are needed, especially to assess to role of combining therapies that may play an increasing role in the future. Considering the absence of reported pancreatic metastases from ICC, achieving an EUS-FNA in order to get a diagnosis was probably the correct strategy. Regarding the lungs nodules we decided to perform a video-assisted thoracoscopic surgery resection although they were highlighted on the PET/CT. Many other causes could explain lung nodules in a young Chinese patient. Therefore we needed a precise diagnosis of the lesion to decide whether the patient could be candidate to surgery or to systemic therapy. Yoon et al[25] reported a case of cholangiocarcinoma that metastasized to the pancreas, however they did not reported whether it was an intrahepatic, hilar or extrahepatic one[25].
In conclusion, the present case report describes a recurrence of intrahepatic cholangiocarcinoma in lungs and pancreas in a patient who underwent liver resection approximately 4 years previously. This is the first report of pancreatic metastasis from ICC.
This case report was showed at the Swiss Congress of Surgery (Bern, June 2013) and the French Congress of Surgery (Paris, October 2013).
A 24-year-old woman was referred for a liver mass.
No symptom, no jaundice. Abdominal palpation was unremarkable with a negative Murphy’s sign.
Hepatocellular carcinoma.
Laboratory tests were perfectly unremarkable.
A computed tomography scan with nonionic contrast confirmed a mass within segments VI-VII measuring 7.2 cm × 6.0 cm. No lesion was observed in the lungs and her pancreas appeared normal.
The tumor consisted of moderately-differentiated intrahepatic cholangiocarcinoma.
Chemotherapy with Gemcitabine/Cisplatin.
Intrahepatic cholangiocarcinomas (ICC) are malignant tumors arising from the intrahepatic bile ducts that frequently recur after resection.
The present case report describes a recurrence of intrahepatic cholangiocarcinoma in lungs and pancreas in a patient who underwent liver resection approximately 4 years previously. This is the first report of pancreatic metastasis from ICC.
This article shows the risk for intrahepatic cholangiocarcinoma to metastasize to the pancreas.
P- Reviewers: Komuta M, Mott JL, Wang HLL S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 344] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 3. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 4. | Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 5. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 6. | Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Canc Netw. 2009;7:423-427. [PubMed] |
| 8. | Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, Guy SR, Sheiner PA, Miller CM, Schwartz ME. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg. 1998;187:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Valverde A, Bonhomme N, Farges O, Sauvanet A, Flejou JF, Belghiti J. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Shirabe K, Shimada M, Harimoto N, Sugimachi K, Yamashita Y, Tsujita E, Aishima S. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery. 2002;131:S159-S164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Konstadoulakis MM, Roayaie S, Gomatos IP, Labow D, Fiel MI, Miller CM, Schwartz ME. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Nuzzo G, Giuliante F, Ardito F, De Rose AM, Vellone M, Clemente G, Chiarla C, Giovannini I. Intrahepatic cholangiocarcinoma: prognostic factors after liver resection. Updates Surg. 2010;62:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Friman S. Cholangiocarcinoma--current treatment options. Scand J Surg. 2011;100:30-34. [PubMed] |
| 16. | Yamamoto M, Ariizumi S. Surgical outcomes of intrahepatic cholangiocarcinoma. Surg Today. 2011;41:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 18. | Sotiropoulos GC, Miyazaki M, Konstadoulakis MM, Paul A, Molmenti EP, Gomatos IP, Radtke A, Baba HA, Beckebaum S, Brokalaki EI. Multicentric evaluation of a clinical and prognostic scoring system predictive of survival after resection of intrahepatic cholangiocarcinomas. Liver Int. 2010;30:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 21. | Zhou HB, Wang H, Li YQ, Li SX, Wang H, Zhou DX, Tu QQ, Wang Q, Zou SS, Wu MC. Hepatitis B virus infection: a favorable prognostic factor for intrahepatic cholangiocarcinoma after resection. World J Gastroenterol. 2011;17:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Marasinghe JP, Karunananda SA, Angulo P. Cholangiocarcinoma in pregnancy: a case report. J Obstet Gynaecol Res. 2008;34:635-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Balderston KD, Tewari K, Azizi F, Yu JK. Intrahepatic cholangiocarcinoma masquerading as the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count) in pregnancy: case report. Am J Obstet Gynecol. 1998;179:823-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA. Liver disease in pregnancy. Lancet. 2010;375:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Yoon WJ, Ryu JK, Kim YT, Yoon YB, Kim SW, Kim WH. Clinical features of metastatic tumors of the pancreas in Korea: a single-center study. Gut Liver. 2011;5:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |