Published online Mar 27, 2014. doi: 10.4240/wjgs.v6.i3.51
Revised: January 5, 2014
Accepted: February 16, 2014
Published online: March 27, 2014
Processing time: 117 Days and 13.9 Hours
Sclerosing encapsulating peritonitis (SEP) is a rare disease entity, in which the small intestine becomes encased and mechanically obstructed by a dense, fibrotic membrane. The disorder is characterized as either primary (idiopathic) or secondary to other causes. The idiopathic cases of SEP, which lack any identifiable etiology according to clinical, radiological and histopathological findings, are also reported under the designation of abdominal cocoon syndrome. The most frequent presenting symptoms of all SEP cases are nausea, vomiting, abdominal distention and inability to defecate, all of which are associated with the underlying intestinal obstruction. Persistent untreated SEP may advance to intestinal perforation, representing a life-threatening condition. However, preoperative diagnosis remains a particular clinical challenge, and most diagnoses are confirmed only when the typical fibrous membrane encasing the small intestine is discovered by laparotomy. Here, we report the clinical presentation of an 87-year-old male with signs of intestinal obstruction and the ultimate diagnosis of concurrent abdominal cocoon, right incarcerated Meckel’s diverticulum, and gastrointestinal perforation in laparotomy.
Core tip: Abdominal cocoon syndrome, also known as idiopathic sclerosing encapsulating peritonitis, is a rare disease entity, in which the small intestine becomes encased and mechanically obstructed by a dense, fibrotic membrane. While some patients with cocoon syndrome remain asymptomatic, the majority experience gastrointestinal symptoms, including recurrent attacks of acute, sub-acute or chronic gastrointestinal obstruction, weight loss, loss of appetite, and development of a palpable abdominal mass. Herein, we describe an elderly patient who presented with signs of intestinal obstruction and who was diagnosed with concurrent abdominal cocoon, right incarcerated Meckel’s diverticulum, and gastrointestinal perforation by exploratory laparotomy.
- Citation: Akbulut S, Yagmur Y, Babur M. Coexistence of abdominal cocoon, intestinal perforation and incarcerated Meckel’s diverticulum in an inguinal hernia: A troublesome condition. World J Gastrointest Surg 2014; 6(3): 51-54
- URL: https://www.wjgnet.com/1948-9366/full/v6/i3/51.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i3.51
Sclerosing encapsulating peritonitis (SEP) is a rare structural abnormality whereby the small intestine becomes encased (or cocooned) by a dense fibrocollagenous membrane that causes intestinal obstruction[1,2]. First described in 1907 as “peritonitis chronica fibrosa incapsula” by Owtschinnikow[1,3-7], subsequent case reports have described the disease condition as primary (idiopathic) or secondary, depending on the underlying etiological causes. The idiopathic form is also reported under the alternative descriptive name of “abdominal cocoon syndrome”[1,3,4,8,9].
The most common clinical signs and symptoms of SEP include nausea, vomiting, abdominal distention and inability to defecate, all of which are indicative of gastrointestinal obstruction. Rare cases of SEP manifest the severe complication of perforation. The relatively non-specific nature of the common symptomology makes preoperative diagnosis a clinical challenge, and many cases are only diagnosed during laparotomy[3,6]. In this case report, we describe an elderly patient who presented with signs of intestinal obstruction and who was diagnosed with concurrent abdominal cocoon, right incarcerated Meckel’s diverticulum, and gastrointestinal perforation by exploratory laparotomy.
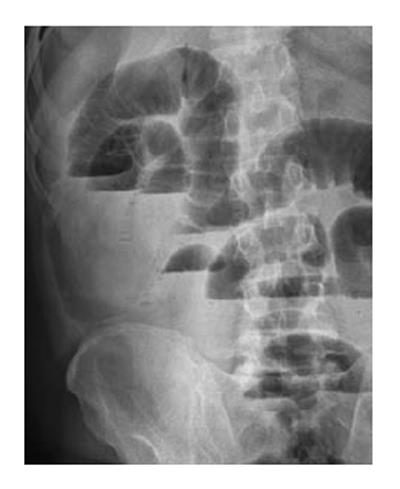
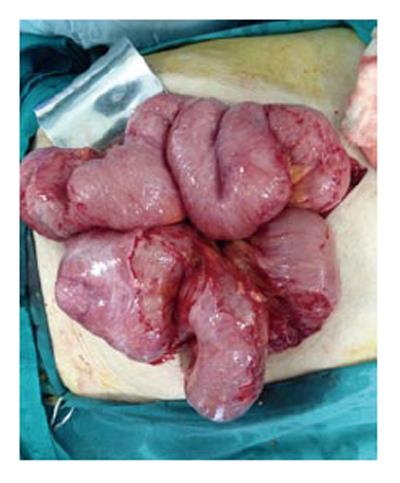
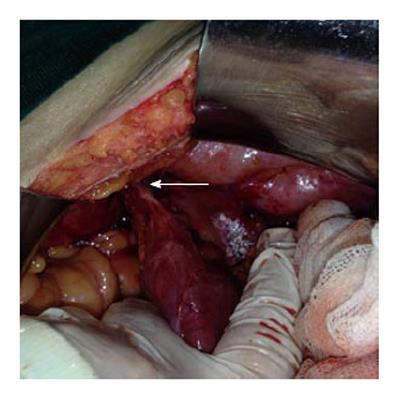
An 87-year-old male patient presented to our emergency department upon referral from an outside center for management of severe abdominal pain, nausea, vomiting and inability to defecate for 3 d. The patient’s medical history was generally unremarkable, with hypertension and bronchial asthma the only major afflictions. Physical examination findings were distended abdomen, sunken eyes, dry mucosa, and vital signs consistent with hypovolemia and sepsis (blood pressure: 100/60 mmHg; pulse: 90 beats per minute; body temperature: 38.1 °C). In addition, severe rebound tenderness was observed in all abdominal quadrants, with the most severe being in the right lower quadrant. Findings from laboratory tests were white blood cell count of 13900/μL (neutrophils: 83.2%), hemoglobin level of 11.4 g/dL, blood urea nitrogen level of 60 mg/dL, and creatinine level of 1.5 mg/dL. Abdominal imaging examination revealed diffuse air-fluid levels (by X-ray; Figure 1) and free fluid in the pelvis and right lower quadrant, as well as dilatation and edema in all intestinal segments (by ultrasonography, United States). Exploratory laparotomy was performed according to the initial diagnosis of perforation, mechanical bowel obstruction due to tumor, and mesenteric vascular disease. All intestinal segments from 60 cm distal to the Treitz ligament to the ileocecal valve were found to be dilated and encapsulated (Figure 2). Meckel’s diverticulum, at the 60 cm proximal to ileocecal valve, was incarcerated into the right inguinal canal. This clinicopathologic condition is also referred to as Littre hernia (Figure 3). Some intestinal segments at 100 cm proximal to the ileocecal valve were conglomerated and adhered to the retroperitoneum. In addition, a 1 cm perforation immediately proximal to the conglomerated segment and an abscess located posterior to the conglomerated segment were detected. The intra-operative management was initiated by performing decapsulation and adhesiolysis of all encapsulated segments, and followed by freeing of the incarcerated intestinal segments and closing of the mouth of the hernia sac. Then, the conglomerated and perforated ileal segment was resected and the abdominal cavity was irrigated. A side-to-side ileoileal anastomosis was constructed using an Endo-GIA stapler (Covidien, Dublin, Ireland), and a loop ileostomy was constructed at 40 cm proximal to the anastomosis site. Post-operative recovery was uncomplicated and the patient was discharged to home at 12 d after the surgery. The histopathologic examination of the excised peritoneal capsule showed proliferation of the fibroconnective tissue with signs of a non-specific inflammatory reaction. Diagnosis of SEP was established based on the intraoperative view, results from histopathologic examination, and findings from other clinical and biochemical analyses.
SEP is a disease entity characterized by partial or complete encasement of small intestine by a thick fibrotic membrane[1,5]. This fibrocollagenous membrane may extend to encase other proximal organs as well, such as stomach, liver and large intestine; in some forms of the disease, this extension of the encasing membrane can cause segregation of the intraperitoneal organs (as if they were extraperitoneal)[8]. Although epidemiological studies of SEP have not fully elucidated the precise etiologic profile of primary/idiopathic or secondary SEP[4], they have revealed a trend in incidence involving young females living in temperate geographic zones. It has been speculated that infections of the Fallopian tubes or retrograde menstruation may be related to disease onset and progression[7]. In addition, incidence of SEP is not infrequent in patients with ambulatory peritoneal dialysis, suggesting that this condition may represent an etiology of secondary SEP[6,10]. Other cases of SEP have been reported in patients with abdominal tuberculosis, sarcoidosis, gastrointestinal malignancies, systemic lupus erythematosus, familial Mediterranean fever, with fibrogenic foreign materials, undergoing beta-blocker (practolol) therapy[4,7,9-12], fitted with ventriculoperitoneal and peritoneovenous shunts, recipients of orthotopic liver transplantation, and suffering from recurrent peritonitis attacks[6]. The current patient, described herein, had no chronic disease history and normal test results from biochemical (erythrocyte sedimentation rate) and microbiological (blood and peritoneal fluid culture, and PPD skin test) assays. Therefore, we considered that this case was likely primary SEP.
While some SEP patients remain asymptomatic, the majority experience gastrointestinal symptoms, including recurrent attacks of acute, sub-acute or chronic gastrointestinal obstruction, weight loss, loss of appetite, and a palpable abdominal mass[1,3,6,8]. Additionally, some of the SEP patients with severe abdominal distention also have ascites[3]. Gastrointestinal perforation is a relatively rare complication of SEP. To our knowledge only one case of SEP-related perforation has been reported, and the etiology was evidenced as tuberculosis[12]. The current case herein also had an ileal perforation, but the absence of any clear etiologic factor (primary SEP) makes this case unique in the literature.
SEP is suspected according to the gastrointestinal clinical signs coupled with suggestive findings from physical examination, biochemical testing, and radiological analyses [i.e., X-ray, barium studies, United States, computed tomography (CT)]. Unfortunately, preoperative diagnosis of SEP remains a challenge and intraoperative findings and histopathological data are required for a definitive diagnosis[9]. Malignancy (particularly colorectal tumor) is the first differential diagnosis considered for individuals with advanced age, unremarkable medical history, physical symptoms of intestinal obstruction, and abdominal X-ray detection of air-fluid levels[1,2,6]. Furthermore, ulcerative perforation, septic peritonitis, and tuberculotic peritonitis encapsulans should be considered in the differential diagnosis.
Barium-enhanced imaging is not always possible in patients with marked intestinal obstruction. Abdominal US can demonstrate a dilated intestinal segment, free fluid accumulation, and the status of the peritoneal membrane (when sufficiently thickened)[4]. Abdominal United States examination of the current case showed free fluid in the pelvis and right lower quadrant, as well as dilatation and edema in all intestinal segments. Nonetheless, CT is considered the best imaging modality for diagnostic purposes, as it can show thickened peritoneum and mesentery as well as capsulated intestinal loops[13]. The typical CT finding for SEP is intestinal loops conglomerated at the midline and encased by a dense mantle without contrast uptake[5,6,13]. Since an urgent laparotomy had to be performed on our patient to address the severe distention, we did not have the opportunity to carry out a CT scan.
The typical finding of SEP is a bobbin-like appearance of the intestine, which results from the partial or whole encasement of intestinal segments by the thick, dense membrane[9]. Upon histopathological examination, the encapsulating membrane appears as a thickened and inflamed vascular fibrocollagenous tissue, with infiltrating lymphocytes and plasma cells[5]. The efficacy and safety of surgical treatment for cases that have a surgical indication and are confirmed by laparotomy remain unknown. Review of the literature suggests that many surgeons favor a minimally invasive approach to treating SEP[6], possibly because the sclerotic membranes on intestinal surfaces (and also between segments) can be easily removed in mild cases without marked intestinal obstruction. However, it is almost impossible to remove the sclerotic membrane successfully in patients with complete intestinal obstruction.
Clinical suspicion and early diagnosis of SEP are crucial to disease and treatment outcome[7]; overly aggressive surgical intervention in SEP cases with severe adhesions may cause multiple perforations. In summary, the basic approach of surgical treatment should include freeing of adhesions, total excision of the membrane, or partial intestinal resection when necessary[9]. It has been reported that the morbidity and mortality rates are higher in patients undergoing intestinal resection[9].
Entirely by coincidence, several clinical conditions were present simultaneously in the case presented herein. The clinical picture of this patient was made even worse by the collective presence of intestinal perforation, a focus of interloop abscess extending to retroperitoneum, and a Meckel’s diverticulum incarcerated into the right inguinal canal. Fortunately, the patient experienced an uncomplicated recovery following the surgical treatment, at least partially due to the well-managed clinical care that was given.
In conclusion, SEP is a rare disease entity causing intestinal obstruction. Preoperative diagnosis is considerably difficult to make, and the majority of previously reported cases have been diagnosed incidentally during laparotomy[11]. Some imaging methods may help clinicians to make the diagnosis of suspected cases. Surgery is an important treatment modality for SEP, but the dissections must be made carefully to free the small intestine and resect the affected tissues and to allow a complete cure[3,6].
Clinical symptoms included severe abdominal pain, nausea, vomiting and inability to defecate.
Mechanical small bowel obstruction.
Gastrointestinal malignancy, perforation, herniation, and mesenteric vascular disease.
Laboratory analysis showed leukocytosis (neutrophils: 83.2%) and elevated levels of renal function markers.
Abdominal X-ray examination showed diffuse air-fluid levels and abdominal ultrasonography revealed free fluid in the lower right quadrant and pelvis, as well as dilatation and edema in all intestinal segments.
Histopathologic examination of the excised peritoneal capsule showed proliferation of fibroconnective tissue with signs of a non-specific inflammatory reaction. The diagnosis of abdominal cocoon syndrome was established according to the intraoperative view and by ruling-out any other causes.
The operative treatment included decapsulation, adhesiolysis, partial small bowel resection, side-to-side ileoileal anastomosis (using an endoscopic stapler), and a loop ileostomy (opened at 40 cm proximal to the anastomosis site).
Early diagnosis of abdominal cocoon syndrome is crucial to disease and treatment outcome; overly aggressive surgical intervention in cocoon cases may cause severe adhesions. The basic approach of surgical treatment should include freeing of adhesions, total excision of the membrane, or partial intestinal resection when necessary, as in our case.
Sclerosing encapsulating peritonitis, also known abdominal cocoon syndrome, is a rare structural abnormality whereby the small intestine becomes encased (or cocooned) by a dense fibrocollagenous membrane that causes intestinal obstruction.
This paper demonstrates coexistence of abdominal cocoon, intestinal perforation and incarcerated Meckel’s diverticulum in an inguinal hernia, and considered to be well written.
P- Reviewers: Afshar S, Guan YS, Hotta T S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Kumar J, Garg A, Chowdhury V, Prakash A, Singh S. Abdominal cocoon--a rare case of intestinal obstruction. A report of two cases. Arab J Gastroenterol. 2012;13:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Wani I, Ommid M, Waheed A, Asif M. Tuberculous abdominal cocoon: original article. Ulus Travma Acil Cerrahi Derg. 2010;16:508-510. [PubMed] |
| 3. | Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012;18:1999-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (2)] |
| 4. | Solak A, Solak İ. Abdominal cocoon syndrome: preoperative diagnostic criteria, good clinical outcome with medical treatment and review of the literature. Turk J Gastroenterol. 2012;23:776-779. [PubMed] |
| 5. | Rastogi R. Abdominal cocoon secondary to tuberculosis. Saudi J Gastroenterol. 2008;14:139-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol. 2007;13:3649-3651. [PubMed] |
| 7. | Lin CH, Yu JC, Chen TW, Chan DC, Chen CJ, Hsieh CB. Sclerosing encapsulating peritonitis in a liver transplant patient: a case report. World J Gastroenterol. 2005;11:5412-5413. [PubMed] |
| 8. | Babbitt BP, Matsueda G, Haber E, Unanue ER, Allen PM. Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci USA. 1986;83:4509-4513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Serter A, Kocakoç E, Çipe G. Supposed to be rare cause of intestinal obstruction; abdominal cocoon: report of two cases. Clin Imaging. 2013;37:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Térébus Loock M, Lubrano J, Courivaud C, Bresson Vautrin C, Kastler B, Delabrousse E. CT in predicting abdominal cocoon in patients on peritoneal dialysis. Clin Radiol. 2010;65:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Gadodia A, Sharma R, Jeyaseelan N. Tuberculous abdominal cocoon. Am J Trop Med Hyg. 2011;84:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Bani-Hani MG, Al-Nowfal A, Gould S. High jejunal perforation complicating tuberculous abdominal cocoon: a rare presentation in immune-competent male patient. J Gastrointest Surg. 2009;13:1373-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ndiaye AR, Mbengue A, Soko TO, Diémé EP, Diagne NM, Diouf CT, Fall A, Fall F, Diop Y, Diakhaté IC. Idiopathic sclerosing encapsulating peritonitis: a case in an adolescent girl. Diagn Interv Imaging. 2012;93:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |