Published online Feb 27, 2014. doi: 10.4240/wjgs.v6.i2.14
Revised: October 30, 2013
Accepted: January 13, 2014
Published online: February 27, 2014
Processing time: 190 Days and 5.7 Hours
Although many studies have focused on the preoperative risk factors of anastomotic leakage after colorectal surgery (CAL), postoperative delay in diagnosis is common and harmful. This review provides a systematic overview of all available literature on diagnostic tools used for CAL. A systematic search of literature was undertaken using Medline, Embase, Cochrane and Web-of-Science libraries. Articles were selected when a diagnostic or prediction tool for CAL was described and tested. Two reviewers separately assessed the eligibility and level of evidence of the papers. Sixty-nine articles were selected (clinical methods: 11, laboratory tests: 12, drain fluid analysis: 12, intraoperative techniques: 22, radiology: 16). Clinical scoring leads to early awareness of probability of CAL and reduces delay of diagnosis. C-reactive protein measurement at postoperative day 3-4 is helpful. CAL patients are characterized by elevated cytokine levels in drain fluid in the very early postoperative phase in CAL patients. Intraoperative testing using the air leak test allows intraoperative repair of the anastomosis. Routine contrast enema is not recommended. If CAL is clinically suspected, rectal contrast-computer tomography is recommended by a few studies. In many studies a “no-test” control group was lacking, furthermore no golden standard for CAL is available. These two factors contributed to a relatively low level of evidence in the majority of the papers. This paper provides a systematic overview of literature on the available tools for diagnosing CAL. The study shows that colorectal surgery patients could benefit from some diagnostic interventions that can easily be performed in daily postoperative care.
Core tip: Postoperative delay in diagnosis of colorectal anastomotic leakage is common and harmful. This paper provides a systematic overview of literature on the available tools for diagnosing colorectal surgery. The study shows that colorectal surgery patients could benefit from some diagnostic interventions that can easily be performed in daily postoperative care.
- Citation: Daams F, Wu Z, Lahaye MJ, Jeekel J, Lange JF. Prediction and diagnosis of colorectal anastomotic leakage: A systematic review of literature. World J Gastrointest Surg 2014; 6(2): 14-26
- URL: https://www.wjgnet.com/1948-9366/full/v6/i2/14.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i2.14
Anastomotic leakage is the most frequent major adverse event after colorectal surgery and remains a large burden for patients and surgeons[1]. Despite evolutions in stapling techniques and operation modalities, incidence of anastomotic leakage after colorectal surgery (CAL) has not decreased over the last decade[1,2]. In the abundant literature on CAL, figures on incidence vary widely, most probably because many studies did not apply the unequivocal definition of CAL that has been available since 2010[3,4]. Clinical signs of CAL before the fifth postoperative day (POD) are uncommon, and most studies described a mean POD of 8 d for CAL to become clinically apparent. However, some studies even show that CAL is diagnosed at mean POD 12[5,6]. Short-term morbidity and mortality, as well as detrimental long-term effects, such as permanent stoma, might be reduced if CAL is detected and treated in an early phase[7]. Many studies have focused on preoperative risk factors, such as age, sex, neoadjuvant therapy, emergency surgery and distance to the anal verge, and should enable an estimation of risk of postoperative CAL[8-11]. Despite this caution, delay in diagnosis is common and has been described to be caused by false negative radiological investigation and intervening weekends[12]. This study was designed to provide colorectal surgeons with a systematic review of the predictive value of the diagnostic techniques for detection of CAL that are currently described in literature.
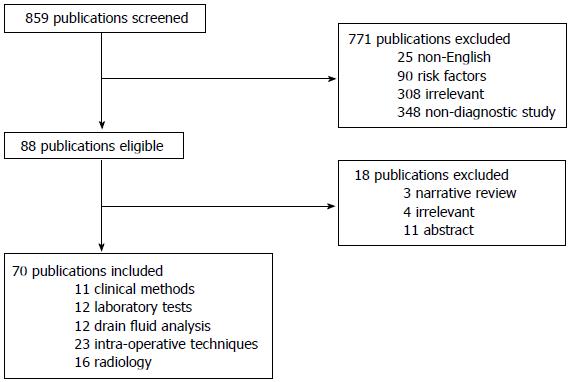
A systematic search of literature was undertaken using Medline, Embase, Cochrane and Web-of-Science libraries. No limitations for year of publication were applied. Search terms were: anastomosis, leakage, dehiscence, colorectal, rectum, resection, anterior resection, diagnosis, sensitivity, specificity, prediction, forecasting, monitoring. The search was restricted to publications in English and French. Full search syntax is shown in Addendum and was carried out lastly on 15 October, 2012. All references in eligible articles were screened for additional publications. Articles were retrieved according to the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses guidelines (Figure 1).
Articles were selected if a diagnostic tool or prediction model for CAL was described and tested, preferably using a reference. Furthermore, definition of CAL was required. If an article described more than one diagnostic tool, it was included for all the tools that were addressed, with the exception of the technique serving as reference/golden standard.
Studies were excluded if they reported on risk prediction of other complications than CAL. The included anastomosis were ileo-colic, colo-colic, colorectal and colo-anal. Total coloproctectomy with ileal pouch anal anastomosis was excluded since etiology, diagnosis and treatment are very different from the types of anastomosis mentioned before. Moreover, studies on risk factors for CAL and randomized trials studying treatment modalities were excluded, as were presentations, experimental studies, narrative reviews and letters to the editor.
For all eligible studies, a standard data extraction form was filled in and the following data were extracted: study design, number of patients, percentage of clinically important CAL, diagnostic tool and main results. If published, sensitivity, specificity, positive predictive value and negative predictive value were noted, or, if possible, calculated. If stated, the POD of CAL diagnosis was recorded. Furthermore, two authors (Daams, Wu) separately determined the level of evidence for validation studies according to the Levels of Evidence 2011 from the Centre for Evidence Based Medicine. In case of inconsistencies, agreement was accomplished by discussion.
The abstracts of a total of 859 articles were screened separately by 2 authors (Daams, Wu) for eligibility. Of these article, 771 were excluded, either for being written in a different language than French and English (n = 25), or for description of preoperative risk factors for CAL (n = 90), or due to irrelevance (n = 308), or because they described a patient cohort or randomized trial or experimental studies, or for other reasons than early detection of CAL (n = 348). This resulted in 88 articles, 18 of which were excluded after full text examination, either for being a narrative review (n = 3), or abstract (n = 11), or due to irrelevance (n = 4).
The remaining 70 articles were included and subdivided into 5 groups, according to type of method used. Two studies were included in two different groups, some studies related to more than one diagnostic tool from one category. (1) Clinical methods: Eleven articles focused on clinical methods, such as the value of physical examination (n = 1), the correlation between clinical symptoms and CAL (n = 5), the application of CAL risk scores (n = 2) or the direct postoperative prediction of the risk of CAL by the surgeon (n = 3); (2) laboratory tests: Twelve articles related to the correlation between CAL and postoperative levels of cytokines (n = 1), C-reactive protein (CRP, n = 10) or coagulation parameters (n = 1); (3) drain fluid analysis: Twelve articles related to diagnosis of CAL by analysing peritoneal drain fluid, in one case using two different methods in one study. The articles focussed on macroscopic findings of drain production (n = 2) or on drain fluid analysis of cytokine levels (n = 6), lipopolysaccharides levels (n = 1) or lysozym levels (n = 1). One article addressed the topic of intramucosal pH-measurement, and two articles focused on microdialysis of the peritoneal cavity; (4) intra-operative techniques: Twenty-three articles investigated the correlation between preoperative assessment of the anastomosis and CAL, using one or more of the following techniques: air/methylene blue leak test (ALT, n = 13), intraoperative endoscopy (IOE, n = 4), Doppler ultrasound (n = 2), tissue oxygen tension measurement (n = 1), intraoperative inspection of marginal artery bleeding (n = 1), laser fluorescence angiography (LFA, n = 1) and near infra-red/visible light spectroscopy (n = 2); and (5) radiology: Sixteen studies evaluated the accuracy of one or more of the following radiological techniques in detecting CAL: computer tomography (CT, n = 7), water-soluble contrast enema (WSCE, n = 10) and plain X-ray (n = 2).
Table 1 gives an overview of the main results of the eleven included studies. Three studies described direct postoperative CAL risk prediction by the surgeon. Two studies described direct postoperative assessment by the surgeon as valuable[13,14]. Karliczek et al[15] prospectively studied subjective assessment of the risk of CAL by the surgeon directly after surgery. Low predictive values were found, with a sensitivity of 62% and a specificity of 52% for low rectal anastomosis.
| Author | Type ofstudy | Loe | n (CAL/non-CAL) | Colorectal/rectum | Stapled/handsewn anastomosis | Study subject/tool | Se | Sp | PPV | NPV | ROC | Main outcome | |
| Dekker et al[22] | Pro | 3b | 10/121 | Colorectal | ? | Leakage score | - | - | - | - | 0.95 | OR = 1.74 for leakage score predictive of CAL | |
| den Dulk et al[23] | Pro | 2b | 21/223 | Colorectal | Both | Leakage score | - | - | - | - | - | Delay of treatment reduced from 4 d to 1.5 d | |
| Sutton et al[18] | Pro | 3b | 22/398 | Colorectal | ? | Clinical symptoms | 0.33 | 0.97 | 0.59 | 0.93 | Over 40% of patients with cardiac event has CAL | ||
| Haase et al[19] | Pro | 4 | 3/40 | Colorectal | ? | Clinical symptoms | - | - | - | - | - | No difference in heart rate variability between CAL and non-CAL | |
| Ghariani et al[17] | Retro | 3b | 23/314 | Colon | ? | Clinical symptoms | - | - | - | - | - | Respiratory, neurological disorders and bloating precipitate CAL | |
| Bellows et al[16] | Retro | 3b | 25/311 | Colorectal | ? | Clinical symptoms | Respiratory symptoms | 0.52 | 0.84 | 0.22 | 0.95 | - | Respiratory, neurological disorders and abdominal pain and distension precipitate CAL |
| Neurology symptoms | 0.24 | 0.97 | 0.4 | 0.94 | - | ||||||||
| Abdominal pain and distension | 0.52 | 0.83 | 0.21 | 0.95 | - | ||||||||
| Nesbakken et al[20] | Pro | 3b | 5/56 | Rectum | ? | Clinical symptoms | Daily assessment by surgeon | 0.50 | 0.89 | 0.5 | 0.89 | - | 50% of CAL is silent |
| Tang et al[21] | Pro | 3b | 10/195 | Rectum | Both | Digital rectal examination | 0.98 | - | - | - | - | As valuable as WSCE before stoma closure | |
| Pettigrew et al[13] | Pro | 3b | 28/113 | Colorectal and general | ? | Risk prediction by surgeon | 0.38 | 0.91 | 0.56 | 0.82 | Highest predictive value for postop surg assessment | ||
| Makela et al[14] | Retro | 3b | 44/88 | Rectum | Both | Risk prediction by surgeon | - | - | - | - | - | In 86% of pts with > 3 risk factors CAL occurs | |
| Karliczek et al[15] | Pro | 3b | 26/191 | Colorectal | ? | Risk prediction by surgeon | High anastomosis | 0.38 | 0.46 | - | - | - | Low predictive value for prediction of CAL by surgeon |
| Low anastomosis | 0.62 | 0.52 | - | - | - |
Five studies analysed the postoperative clinical course of patients with CAL in comparison to patients with an uncomplicated course. Two retrospective studies noted that occurrence of respiratory and neurological disorders often precede CAL after colonic surgery (OR = 2.8 and 5.3 respectively)[16,17]. One prospective study noted that cardiac disorders preceded CAL in 40% of 22 patients with CAL[18]. A small study reported no differences in heart rate variability between patients with and without CAL[19]. In a prospective study by Nesbakken et al[20], the postoperative assessment of the patient by the surgeon was reported to have high specificity and low sensitivity (91% and 50% respectively). Tang et al[21] investigated the value of digital rectal examination in assessing CAL before stoma closure, and found a sensitivity of 98.4%.
Two Dutch authors developed and applied leakage scores for the detection of CAL. One risk score prospectively combined preoperative and intraoperative items and yielded a twofold higher score in patients with CAL than in patients without CAL[22]. For postoperative clinical course assessment, a standardized leakage score was developed by den Dulk et al[23] attributing points to certain clinical factors, nutritional status and biochemic findings, thus identifying high risk patients. It facilitated the diagnosis of CAL at POD 6, as opposed to POD 8 in a historical control group.
Ten studies investigated the correlation between postoperative levels of CRP and CAL as shown in Table 2[24-28]. Five of them were included in a meta-analysis of 1832 patients by Warschkow et al[24], which did not focus solely on CAL but on all postoperative infectious complications. In all studies, CRP-levels were elevated several days before the diagnosis of CAL was established. Slotwinski and colleagues reported higher levels of soluble-tumour necrosis factor (TNF)-receptor at POD 1 in patients who developed CAL after colorectal surgery[29]. Iversen et al[30] studied levels of markers of coagulation and fibrinolysis in patients with CAL showed elevated levels 5-6 PODs before clinical onset of CAL compared to patients without leakage.
| Author | Type of study | Loe | n (CAL/non-CAL) | Colorectal/rectum | Stapled/handsewn anastomosis | Study subject/tool | Cut-off value | Se | Sp | PPV | NPV | ROC | Main outcome | OnsetCAL(POD) |
| Slotwinksi et al[29] | Pro | 3b | 2/16 | Colorectal | ? | sTNF-R1, IL-1RA/-6/-8/-10, CRP | - | - | - | - | - | - | TNF higher at POD 1 in CAL | ? |
| Iversen et al[30] | Pro | 3b | 17/341 | Colorectal | Both | s-Fibrin, TAT-complex, PT-f1/-2 | - | - | - | - | - | - | PT-f1/-2, TAT-complex, s-Fibrin higher at POD 1/2 in CAL | 7 |
| Woeste et al[25] | Retro | 3b | 26/342 | Colorectal | Both | CRP | - | - | - | - | - | - | CRP higher from POD 3 to POD 7 in CAL | 8,7 |
| Warschkow et al[24] | Meta | 3a | ?/1832 | Colorectal | Both | CRP | 135 mg/L at POD 4 | 0.680 | 0.830 | 0.560 | 0.89 | - | CRP < 135 mg/L at POD 4 discharge is safe | ? |
| Kornerin et al[24] | Retro | 3b3 | 18/231 | Colorectal | Both | CRP | 190 mg/L at POD 3 | 0.820 | 0.730 | - | - | 0.820 | Persisting elevation of CRP is indicative for CAL | 8 |
| Mackayin et al[24] | Pro | 3b3 | 5/160 | Colorectal | ? | CRP | 145 mg/L at POD 4 | 0.850 | 0.860 | 0.610 | 0.96 | - | CRP > 145 mg/L at POD 4 is highly predictive for CAL | ? |
| Ortegain et al[24] | Pro | 3b3 | 21/133 | Colorectal | Both | CRP | 125 mg/L at POD 4 | 0.820 | 0.960 | - | - | - | CRP > 125 mg/L at POD 4 discharge is not safe | 6 |
| Welschin et al[24] | Pro | 3b3 | 22/961 | Rectum | Staples | CRP | 140 mg/L at POD 3 | 0.802 | 0.812 | 0.862 | - | - | Persisting elevation of CRP is indicative for CAL | 8 |
| Warschkowin et al[24] | Retro | 3b3 | 89/1115 | Colorectal | ? | CRP | 143 mg/L at POD 4 | 0.750 | 0.710 | 0.190 | 0.97 | - | Use CRP as screening at POD 4 | 9 |
| Platt et al[26] | Pro | 3b | 26/454 | Colorectal | Both | CRP | 190 mg/L at POD 3 | 0.772 | 0.802 | - | - | 0.892 | CRP at POD 3 is useful for predicting CAL | 6-8 |
| Matthiessen et al[27] | Pro | 3b | 9/33 | Rectum | ? | CRP | - | - | - | - | - | - | CRP higher from POD 2 in CAL | 8 |
| Almeida et al[28] | Retro | 3b | 24/149 | Colorectal | ? | CRP | 140 mg/L at POD 3 | 0.780 | 0.860 | - | - | - | CRP sign higher from POD 2 in CAL | 7 |
Table 3 shows twelve studies on drain fluid analysis. Six out of twelve studies investigated cytokine levels after colorectal surgery, mainly focussing on interleukin (IL)-6, IL-10 and TNF-α. In 4 of these studies, patients after colorectal surgery who developed CAL at POD 5-20 had elevated cytokine levels from POD 1 onwards[31-34]. One study reported the same phenomenon, but the onset of increased cytokine levels was POD 3[35]. Another study did not find a relation between CAL and levels of IL-6 and TNF-α[36]. In two studies describing the technique of microdialysis, local signs of ischemia were measured before CAL became clinically apparent in some patients, although both studies also describe patients with CAL who showed no preceding abnormal microdialysis values[33,37]. Macroscopic changes in drain production were examined by Tsujinaka et al[38]. Of 21 patients with CAL, 15 had previous changes in drain content, while other clinical signs were not obvious. Likewise, Eckmann et al[39] found that 80% patients that developed CAL after rectum resection had changes in drain fluid aspect. By measuring intramucosal pH, Millan et al[40] found that the risk of CAL was 22 times higher when juxta-anastomotic intramucosal pH was below 7.28. In a small study, intraperitoneal levels of lipopolysaccharides were elevated from POD 3 in patients with CAL, while CAL was only clinically evident at mean POD 6, 7[41]. By contrast, lysozyme activity was not correlated with clinical CAL in another small study[42].
| Aauthor | Type ofstudy | Loe | n (CAL /non-CAL) | Colorectal/rectum | Stapled/handsewn anastomosis | Study subject/tool | Main outcome | Onset CAL (POD) |
| Bertram et al[36] | Pro | 4 | 3/28 | Colorectal | ? | Cytokines | No correlation between IL-6, TNF-alpha and CAL | 5.3 |
| Herwig et al[34] | Pro | 3b | 12/24 | Colorectal | ? | Cytokines | IL-6 and TNF-alpha elevated from POD 1 in CAL | 5.8 |
| Yamamoto et al[35] | Pro | 3b | 7/90 | Colorectal | Stapled | Cytokines | IL-1beta, IL-6, TNF-alpha elevated from POD 3 in CAL | 5-8 |
| Ugras et al[32] | Pro | 3b | 4/34 | Colorectal | Both | Cytokines | IL-6, IL-10, TNF-alpha elevated from POD 1 in CAL | 6 |
| Fouda et al[31] | Pro | 3b | 8/56 | Rectum | Both | Cytokines | IL-6, IL-10 elevated from POD 1 in CAL, TNF-alpha elevated from POD 2 in CAL | 6 |
| Mattiessen et al[33] | Pro | 3b | 7/23 | Rectum | ? | Microdialysis, cytokines | L/P-ratio elevated at POD 5/6 in CAL; IL-6, IL-10, TNF-alpha elevated from POD 1 in CAL | Early CAL: 6 Late CAL: 20 |
| Ellebaek et al[37] | Pro | 3b | 4/50 | Colorectal | ? | Microdialysis | Mean L/P-ratio higher in CAL, | Early CAL: 5-10 Late CAL: 20 |
| Tsujinaka et al[38] | Pro | 3b | 21/196 | Rectum | Both | Drainproduction | 15/21 Patients with CAL had changes in drain content | 7 |
| Eckmann et al[39] | Retro | 3b | 30/306 | Rectum | Stapled | Drainproduction | 80% of leakages were indicated by drain, 40% of which prior to clinical symptoms | ? |
| Millan et al[40] | Pro | 3b | 6/90 | Colorectal | Stapled | Intramucosal pH | Intramucosal pH < 7.28 on POD1 increases risk of CAL 22 fold | ? |
| Junger et al[41] | Pro | 3b | 3/22 | Colorectal | Both, biodegradable ring | LPS | Excretion of LPS and LPS concentration is higher at POD 3 in CAL | 6,7 |
| Miller et al[42] | Pro | 2b | 2/42 | Rectum | Stapled | Lysozym activity | No correlation between lysozyme activity and CAL | ? |
Table 4 demonstrates the studies on intraoperative techniques to detect CAL. Thirteen studies on peroperative leak tests were evaluated[43-55]. Although these tests facilitate intraoperative repair of the anastomosis or creation of faecal diversion in case of air leakage or methylene blue leakage, postoperative leakage rates were not reduced to 0%. A study by Beard, reported on 18 intraoperative anastomotic corrections, leading to CAL in 3 patients in the “test”-group, compared to 10 patients with CAL in the “no test”-group[43]. As with the air leak test, colonoscopy, performed in 4 studies, led to intraoperative correction of the anastomosis for reasons of leakage and bleeding[52,56-58]. All studies reported low incidences of CAL, although no study compared intraoperative colonoscopy to no intraoperative control. Two studies comparing routine intraoperative colonoscopy to selective use of this technique showed no benefit of routine application of this technique[57,58]. For assessing local anastomotic blood flow, multiple techniques have been described. Ambrosetti et al[59] studied the use of Doppler intraoperatively at the site of the anastomosis, enabling correction of the anastomosis in 10 of 200 patients, leading to CAL in 2 (1%). Vignali et al[60] found that reduced microperfusion at the rectal stump, during creation of a colorectal anastomosis, measured by laser Doppler increased the risk of CAL. In a study by Kudszus et al[61] intraoperative LFA led to 28 intraoperative corrections, an absolute reintervention rate of 4% and reduced hospital stay. Hirano et al[62] studied the application of near infrared spectroscopy of the anastomosis. In their small study, perianastomotic StO2 < 60 mmHg was measured in patients who developed CAL. In a similar study by Karliczek et al[63], using visible light spectroscopy, changes in perianastomotic pO2 before and after creation of the anastomosis had a significant correlation with CAL. One study showed that reduced pO2 in perianastomotic tissue was predictive for CAL, although cut-off values for routine clinical application were lacking[64,65].
| Author | Type of study | Loe | n (CAL/non-CAL | Colorectal/rectum | Stapled/handsewn anastomosis | Test | Testper-formed | Test + | Intra-operative correction | CAL test+ | Test - | CAL test- | Test not per-formed | CALtest not per-formed | Mainoutcome |
| Beard et al[43] | Pro | 1b | 13/145 | Colorectal | Both | ALT | 73 | 18 | 18 | 3 | 55 | 0 | 70 | 10 | ALT and preoperative repair reduce risk of AL |
| Davies et al[44] | Pro | 3b | 4/33 | Rectum | ? | ALT | 33 | 6 | 6 | 1 | 27 | 3 | - | - | LT helpful to reduce leakage rate |
| Dixon et al[45] | Retro | 3b | 2/202 | Rectum | Both | ALT | 119 | 5 | 5 | 0 | 114 | 0 | - | - | Leaks were avoided |
| Gilbert et al[46] | Retro | 3b | 1/21 | Colorectal | Handsewn | ALT | 21 | 5 | 5 | 1 | 16 | 0 | - | - | ALT facilitates IOR |
| Lazorthes et al[47] | Pro | 3b | 3/82 | Colorectal | Stapled, doughnut complete 68 | ALT | 68 | 0 | 0 | 0 | 68 | 3 | - | - | High NPV for ALT |
| Stapled, doughnut incomplete 14 | 14 | 4 | 4 | 0 | 10 | 0 | - | - | |||||||
| Ricciardi et al[48] | Retro | 3b | 48/998 | Colorectal | Both | ALT | 825 | 65 | 65 | 5 | 760 | 29 | 173 | 14 | ALT for leftsided anastomosis |
| Schmidt et al[49] | Pro | 3b | 68/933 | Rectum | Both | ALT | 260 | 47 | 42 | 5 | 213 | 22 | 36 | 4 | Risk of AL is unrelated to ALT |
| Wheeler et al[50] | Pro | 4 | 7/102 | Colorectal | ? | ALT | 99 | 21 | 21 | 2 | 85 | 2 | - | - | LT facilitates IOR |
| Yalin et al[51] | Po | 3b | 1/23 | Colo-rectal | Stapled | ALT | 21 | 5 | 5 | 1 | 16 | 0 | - | - | LT facilitates IOR |
| Griffith et al[54] | Pro | 4 | 2/60 | Colorectal | Stapled | ALT | 60 | 11 | 11 | 0 | 49 | 2 | - | - | ALT facilitates IOR |
| Sakanoue et al[55] | Pro | 3b | 4/70 | Rectum | ? | ALT | 35 | 2 | 2 | 0 | 33 | 0 | 35 | 4 | Useful for intraoperative decision making |
| Smith et al[53] | Pro | 4 | 7/229 | Colon | Both | ALT | 229 | 16 | 16 | 0 | 213 | 7 | - | - | After IOR no CAL occurred |
| Lanthaler et al[56] | Pro | 3b | 6/122 | Colorectal | Stapled | IOE | 73 | 5 | 5 | 0 | 68 | 4 | 49 | 2 | ALT prevents early leak |
| Li et al[57] | Pro | 3b | 2/244 | Rectum | Stapled | IOE | 107 | 11 | 11 | 0 | 96 | 0 | 137, 30 IOC1 | 2/137, 1/30 | Routine IOE and selective IOE equal results |
| Shamiyeh et al[58] | Pro | 3b | 7/253 | Rectum | Stapled | IOE | 85 | 2 | 2 | 0 | 83 | 1 | 253 | 4 | Routine IOE does not reduce CAL |
| Ishihara et al[52] | Pro | 4 | 1/73 | Rectum | Stapled | IOE and ALT | 73 | 4 | 4 | 0 | 69 | 1 | - | - | ALT recommended |
| Ambrosetti et al[59] | Pro | 4 | 2/200 | Colorectal | Both | Doppler ultra-sound | Doppler facilitates IOR | ||||||||
| Vignali et al[60] | Pro | 3b | 8/55 | Colorectal | Stapled | Laser doppler | - | - | - | - | - | - | - | - | Reduction in microperfusion increases risk of CAL |
| Kudszus et al[61] | Retro | 3b | 22/402 | Colorectal | Both | LFA | 201 | 28 | 28 | 8 | - | - | 201 | 15 | LFA reduces reoperation rate for AL, most prominent in handsewn |
| Hirano et al[62] | Pro | 4 | 1/20 | Colorectal | ? | Near infrared spectro-scopy | StO2 < 60% in CAL | ||||||||
| Novell et al[64] | Pro | 3b | 275 | Colorectal | Both | Obser-vation of marginal artery bleeding | Pulsatile flow: lower incidence CAL | ||||||||
| Sheridan et al[65] | Pro | 3b | 5/50 | Colon | ? | Tissue pO2 measurement | Reduced anastomotic pO2 predictive CAL | ||||||||
| Karliczek et al[63] | Pro | 3b | 14/77 | Colorectal | ? | Visible light spectro-scopy | pO2 could predict CAL |
Table 5 demonstrates sixteen studies evaluated several imaging modalities for the detection of CAL. Seven studies in this review used computed tomography (CT) for the detection of CAL[20,66-78]. A prospective study by Nesbakken et al[20] reported a 94% accuracy for 5 patients with CAL out of 56 patients who had received rectum resection. Similarly, Eckmann et al[77] concluded that CT detected 29 of 30 leaks in a group of 305 patients after stapled rectum resection, although no data were presented on the specificity of the technique. Gouya et al[75] even reported an excellent 100% sensitivity and specificity. However CT will only show leakage of intraluminal contrast at the site of the CAL in 10% of the patients[67]. Improved results are achieved with the detection of associated features such like pericolic/pelvic fluid collections[78]. Presacral abnormalities, commonly described as caused by leakage, were found in 70% of the patients without clinical anastomotic leakage[68].
| Author | Type of study | Loe | n (CAL/non-CAL) | Colorectal/rectum | Stapled/handsewn anastomosis | Study tool | Se | Sp | PPV | NPV | Main outcome |
| Eckmann et al[77] | Retro | 3b | 30/306 | Rectum | Stapled | CT | - | - | - | - | 29 of 30 CAL detected by CT |
| Power et al[78] | Retro | 3b | 17/50 | Colorectal | ? | CT | 0.30 | 0.90 | 0.58 | 0.74 | Peri-anastomotic located fluid containing air found in CAL |
| Gouya et al[75] | Retro | 3b | 10/195 | Rectum | ? | CT | - | - | 1.00 | 1.00 | CT has role in predicting CAL |
| DuBrow et al[68] | Retro | 3b | 35/75 | Rectum | ? | CT | - | - | - | - | 30% of pts with CAL have presacral abnormalities |
| Nicksa et al[73] | Retro | 4 | 36 CAL | Rectum | ? | CT | 0.12 | - | - | - | Low percentage true positives |
| Doeksen et al[67] | Retro | 3b | 68/429 | Colorectal | ? | CT | 0.54 | 0.78 | 0.68 | 0.66 | Interobserver variability 10% |
| Nesbakken et al[20] | Pro | 3b | 5/56 | Rectum | ? | CT | 0.57 | 1.00 | - | - | 94% accuracy of CT for detection of CAL |
| Severini et al[74] | Retro | 3b | 12/175 | Rectum | ? | WSCE | - | - | - | - | 2 CAL out of 78 positive WSCE, low predictive value |
| Hoffmann et al[70] | Retro | 3b | 5/51 | Colorectal | Both | WSCE | 0.20 | 0.85 | 0.13 | 0.91 | WSCE not recommended for routine use |
| Markham et al[72] | Retro | 3b | 1/136 | Rectum | Handsewn | WSCE | 1.00 | 0.57 | 0.02 | 1.00 | WSCE no contribution to surgical management |
| Kalady et al[71] | Retro | 3b | 8/211 | Rectum | ? | WSCE | 0.88 | 1.00 | 1.00 | 0.99 | WSCE does not provide additional information |
| Akyol et al[66] | Pro | 3b | 12/233 | Colorectal | Both | WSCE | 0.52 | 0.87 | 0.30 | 0.94 | WSCE provides little useful clinical information |
| Haynes et al[69] | Retro | 3b | 14/117 | Colorectal | Both | WSCE | 0.71 | 0.86 | 0.42 | 0.96 | WSCE not recommended for routine use |
| Gouya et al[75] | Retro | 3b | 10/195 | Rectum | ? | WSCE | - | - | 1.00 | 0,98 | WSCE is recommended for routine use |
| Nicksa et al[73] | Retro | 4 | 36 CAL | Rectum | ? | WSCE | 0.88 | - | - | - | WSCE superior to CT |
| Doeksen et al[67] | Retro | 3b | 68/429 | Colorectal | ? | WSCE | 0.68 | 0.94 | 0.91 | 0.76 | Interobserver variability 13% |
| Nesbakken et al[20] | Pro | 3b | 5/56 | Rectum | ? | WSCE | 0.60 | 1.00 | - | - | 93% accuracy of WSCE for detection of CAL |
| Williams et al[76] | Retro | 4 | 10/31 | Rectum | Stapled | X-ray | 0.90 | 1.00 | 1.00 | 0.95 | Staple line dehiscence in 9/10 patients with CAL |
| Tang et al[79] | Pro | 4 | 2/64 | Colorectal | ? | X-ray | - | - | - | - | Increase free air after POD 5 higher chance CAL |
Ten studies investigated the value of the water-soluble contrast enema in determining CAL, mostly after rectum resection, both in the postoperative phase and before closure of deviating ileostomy[20,66,67,69-75]. All studies described a high degree, in one case even up to 41%[72], of asymptomatic radiological leakage that resolved without therapeutic intervention. In addition, no study performed contrast enemas in the very early postoperative phase (< POD 5) due to the potential risk of complications so that, when performed at POD 7, 8, a clinical leakage concurred with radiological leakage. For these reasons, most studies concluded that routine application of WSCE at POD 7, 8 did not contribute to clinical decision-making or to early detection. In the presence of clinical signs suggestive for CAL, a study by Nesbakken et al[20] described an accuracy of 93% for WSCE in the detection of CAL. Doeksen et al[67] reported a high specificity and positive predictive value of 94% and 91% respectively, with an interobserver variability of 14%.
Two studies investigated the value of plain X-ray. One of these studies reported that increase of subdiafragmatical free air after POD5 increased the likelihood of CAL[76]. The other study, by Williams et al[79], reported that the finding of staple line disruption on plain X-ray was suggestive for CAL.
In this paper, all available evidence on the diagnostic tools for detection of CAL was systematically reviewed, according to the guidelines of the Oxford Centre of evidence based medicine. Diagnostic techniques were appraised for their ability to predict or detect clinically relevant CAL, since this is relevant in daily care for patients directly after colorectal surgery. Early intervention in abdominal sepsis is essential as is shown by the Surviving Sepsis Campaign, emphasizing on source identification and surgical control when possible[80].
Many studies report data on asymptomatic or radiological CAL. However, these data were not included in this review, since asymptomatic CAL, if detected, will be left untreated as a rule. Furthermore, it has a poor correlation with clinically relevant CAL. Theoretically, asymptomatic CAL might prove to be important if the oncologic outcome is studied, since equivocal literature is available showing a higher percentage of local recurrence after CAL[81-83]. To this date, however, the role of asymptomatic CAL in local recurrence is unknown.
Two investigators separately evaluated all eligible studies and a level of evidence was assigned to each of them. Overall, the level of evidence was considered low. This was due to factors that coincide with the problem of CAL. First, in the field of the diagnosis of CAL, no definition of CAL is available, nor is a golden standard[3]. Such a golden standard cannot even be found in relaparotomy during which faecal discharge at the site of the anastomosis is established, since many patients are treated for CAL without direct visualization of the anastomosis during reoperation. Secondly, a major cause of the low level of evidence is the fact that many studies lack a non-test group. Finally, guidelines to determine the level of evidence differ between diagnostic studies and their therapeutic counterparts. Publication bias and reporting bias in particular were estimated to be low, since the primary search yielded many studies with negative results and small numbers of subjects.
Much research has been done on the early detection of leakage after ileoanal pouch reconstruction following total colectomy for inflammatory bowel disease. These studies were excluded from this review, since they comprise more extensive surgery, different types of leakage, other types of pouch failure and different therapy modalities.
Clinical factors are objective and easily available for risk prediction. A few problems, however, occur if surgeons rely solely on clinical factors. First, the influence of individual factors is not exactly known. Secondly, by the time signs of septicaemia occur; patients will be in a worse clinical state at the onset of an often prolonged and onerous therapeutic course. Subjective prognosis of leakage at the moment of finishing the anastomosis was proven to have a limited prognostic value[15]. Objective measurements might be of greater prognostic value, as shown by the Colon Leakage Score, in which the presence of objective risk factors leads to a higher score representing a higher chance of CAL[22]. This leakage score was based on previously identified risk factors and to our knowledge is the first to translate all available literature on risk factors for CAL into an instrument that can easily be implemented in daily practice. In a cohort of 233 patients, using a historical control group of 1066 patients, den Dulk et al[23] developed a similar score system for postoperative clinical evaluation of the colorectal patient. When a high score is found, computer tomography using rectal contrast is warranted. Although this promising method has shown to reduce delay in diagnosis, no information was provided on the prognostic value of this risk score, nor did the study mention the number of CT-scans and concomitant negative results In a study on tracking of surgical site infections (SSI), van Ramshorst et al[84] found that protocolled tracking yields a higher reported incidence of SSI than self-reported detection. We believe that this finding could be applied to the protocolled detection of CAL as described above, as it contributes to increased awareness and early detection.
Little is known about the value of physical examination in relation to CAL, except that digital rectal examination has at least the same prognostic value for low anastomosis as contrast enema prior to stoma reversal.
Many investigators have studied the behaviour of CRP during the subclinical phase of CAL. CRP has the capacity to rise quickly after the onset of an inflammatory stimulus, reaching its highest serum level within 48 h. Since it has a short halftime of around 19 h, a drop in CRP corresponds well with the removal of the stimulus. Most studies investigating CRP used cut-off values of around 120-190 mg/L at POD 3, 4, and all studies in this review showed a reasonable predictive value of CRP for CAL. Drawbacks of all studies described in this review is that the number of included patients per study is rather small and that none of these studies provide a protocol that structurally describes the postoperative clinical examination, the clinical state of the patients during postoperative follow-up and the type of CAL (i.e., faecal peritonitis, juxta-anastomotic abscess, rectovaginal fistula). Despite these drawbacks, we believe that these studies have indeed shown that measurement of CRP is of great importance in detecting CAL in the preclinical phase.
Other laboratory tests like coagulation factors and cytokines show a correlation with occurrence of CAL, but they have been studied sparsely. Since no parameters for their predictive value can be calculated from the available data, there is no basis for incorporating them in the standard postoperative lab tests.
In this review, the results for cytokine levels in peritoneal drain fluid, as biomarkers for local infection, seem promising. In most studies cytokine levels were elevated from POD 1 in patients with CAL compared to patients without CAL. This finding suggests an early onset of local infection in patients with CAL, or at least a more prominent postoperative reaction in this group. It is hypothesised that cytokines are directly elevated postoperatively and will normalise unless infectious complications occur. Most frequently investigated cytokines are IL-1, IL-6, IL-10 and TNF-α.
Although routine drainage after colorectal surgery does not seem to prevent CAL and is omitted in enhanced recovery programs, two studies showed that changes of drain production occur frequently and before clinical symptoms. These interesting findings might justify the routine placement of a drain for the first postoperative days as an indicator for CAL.
Two studies on intraperitoneal microdialysis show, by retrospectively analysing of peritoneal microdialysis samples, that CAL was preceded by changes in local lactate/pyruvate ratio. Although these findings are promising, patient numbers were too low to compute predictive values and cut-off values. Future research should elucidate if prospective, real-time analysis actually leads to early detection and determine whether this technique is cost effective.
For intramucosal pH monitoring, as a measure for mucosal hypoperfusion and subsequent hypoxia, data are limited but promising. The same holds for measurement of lipopolysaccharides, integral components of normal gut flora, and measurements of lysozym in drain fluid, since the studies investigating these biomarkers did neither lead neither to confirmation of these techniques nor to a re-evaluation.
Except for one, all studies evaluating the ALT confirm the importance of this simple intervention. Although not completely eliminating the occurrence of CAL, ALT allows intraoperative revision of the anastomosis, is easy to perform and has a high negative predictive value. Understandably, no studies have been performed that relate a positive ALT without intraoperative repair to CAL. All valuable studies, those that use a no-test control group, show a lower percentage of CAL in the group in which ALT was performed; in two out of four papers this difference was significant.
IOE can, apart from direct visualisation of CAL, be of diagnostic and therapeutical importance if the location of the tumour or of additional lesions is unknown or if anastomotic bleeding occurs. More recently, the routine application of IOE has been studied in comparison to selective IOE. No favourable results in occurrence of CAL were described for routinely performed IOE compared to selective IOE. Apart from the mentioned benefits of IOE, no data are available on the superiority of IOE compared to ALT for intraoperative diagnosis of anastomotic dehiscence. Thus, ALT seems to be favourable to IOE since it is faster, easier and cheaper.
Some authors have attempted to relate anastomotic perfusion parameters to anastomotic leakage. Except for one, all studies are case controlled without reference and have not been repeated. It has not led to clear cut-off values for any of these techniques that seem not very practical in daily current practice. At least one cohort study with a good reference is needed before clinical implementation.
As far as CT with rectal contrast is concerned, only 7 studies could be included. These studies showed large differences in methodology and lacked generally applied definitions. These differences between several studies, especially in CT criteria for CAL, resulted in equivocal results. Intestinal contrast leakage is not regularly depicted with CT in patients with CAL. However CT can accurately depict the associated features of anastomotic leakage such like pericolic/pelvic fluid collections and free air. When these additional criteria were used the accuracy improved dramatically with accuracies varying from 80%-100%.
All six studies that were performed on the subject of WSCE over the last two decades concluded that there is no place for routine application of WSCE. In these studies, WSCE did not have a consistently high positive predictive value, and other techniques, such as digital rectal examination in low rectal anastomosis, appeared to provide at least equal results. Furthermore due to the potential risk of complications no study performed contrast enemas in the very early postoperative phase. This means that, when performed at POD 7-8, clinical CAL concurred with radiological leakage. In addition, radiologic signs of CAL do not correlate with clinical CAL and frequently do not require any form of treatment. Another drawback of WSCE is that the rectally administered contrast has been diluted and there may be not enough remaining pressure to induce contrast leakage in more proximal anastomoses.
Two older studies describe how plain X-rays can be used in assessment of intra-abdominal free air and staple line integrity in the diagnosis of CAL. Although sometimes helpful, modern techniques offer the surgeon much more detailed information on the extend of CAL compared to plain X-rays.
Many studies have been performed in the field of diagnosis of CAL. Many lack a no-test control group and reference; therefore the general level of evidence is relatively low. The air leak test is recommended for intraoperative assessment of CAL. When a leakage score system is used intraoperatively, preoperative preventive measures can be taken. When using a clinical algorithm postoperatively, delay in diagnosis of CAL might be reduced. CRP measurement should be part of postoperative laboratory routine at least at POD 3 and 4, since due to a high negative predictive value patients with an uncomplicated course can be identified. Cytokine measurement among other measurements of peritoneal drain fluid is promising and could justify the routine placement of a juxta-anastomotic drain, while peritoneal microdialysis might develop as minimally invasive peritoneal “smart”-drain. When clinical signs are present, CT with rectal contrast is recommended. CT cannot only to detect CAL but also can be used as a therapeutic instrument for percutaneous drainage of a pericolic/pelvic abscess. We believe that this review reaffirms the importance of early detection of colorectal anastomotic leakage and that it offers colorectal surgeons an overview on easily applicable diagnostic tools to improve early detection.
The authors would like to thank Wichor Bramer, Biomedical Information Specialist at the Medical Library at the ErasmusMC Rotterdam the Netherlands, for his contribution during the collection of data.
P- Reviewers: Akiyoshi T, Rutegard M S- Editor: Cui XM L- Editor: A E- Editor: Wu HL
| 1. | Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999;189:554-559. [PubMed] |
| 2. | Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Bruce J, Krukowski ZH, Al-Khairy G, Russell EM, Park KG. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88:1157-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 510] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 4. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (4)] |
| 5. | Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 454] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 6. | Komen N, Dijk JW, Lalmahomed Z, Klop K, Hop W, Kleinrensink GJ, Jeekel H, Ruud Schouten W, Lange JF. After-hours colorectal surgery: a risk factor for anastomotic leakage. Int J Colorectal Dis. 2009;24:789-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Macarthur DC, Nixon SJ, Aitken RJ. Avoidable deaths still occur after large bowel surgery. Scottish Audit of Surgical Mortality, Royal College of Surgeons of Edinburgh. Br J Surg. 1998;85:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Canelas A, Bun M, Cabo JK, Laporte M, Peczan C, Rotholtz N. Risk factors associated to anastomotic leakage in laparoscopic colorectal surgery. Colorectal Dis. 2010;12:37. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Isbister WH. Study populations and casemix: influence on analysis of postoperative outcomes. Aust N Z J Surg. 2000;70:279-284. [PubMed] |
| 10. | Lai R, Lu Y, Li Q, Guo J, Chen G, Zeng W. Risk factors for anastomotic leakage following anterior resection for colorectal cancer: the effect of epidural analgesia on occurrence. Int J Colorectal Dis. 2013;28:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Warschkow R, Steffen T, Thierbach J, Bruckner T, Lange J, Tarantino I. Risk factors for anastomotic leakage after rectal cancer resection and reconstruction with colorectostomy. A retrospective study with bootstrap analysis. Ann Surg Oncol. 2011;18:2772-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Doeksen A, Tanis PJ, Vrouenraets BC, Lanschot van JJ, Tets van WF. Factors determining delay in relaparotomy for anastomotic leakage after colorectal resection. World J Gastroenterol. 2007;13:3721-3725. [PubMed] |
| 13. | Pettigrew RA, Hill GL. Indicators of surgical risk and clinical judgement. Br J Surg. 1986;73:47-51. [PubMed] |
| 14. | Mäkelä JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum. 2003;46:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis. 2009;24:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Bellows CF, Webber LS, Albo D, Awad S, Berger DH. Early predictors of anastomotic leaks after colectomy. Tech Coloproctol. 2009;13:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Ghariani B, Houissa H, Sebai F. Early diagnosis of anastomotic dehiscence after colonic surgery. Tunis Med. 2011;89:174-178. [PubMed] |
| 18. | Sutton CD, Marshall LJ, Williams N, Berry DP, Thomas WM, Kelly MJ. Colo-rectal anastomotic leakage often masquerades as a cardiac complication. Colorectal Dis. 2004;6:21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Haase O, Langelotz C, Scharfenberg M, Schwenk W, Tsilimparis N. Reduction of heart rate variability after colorectal resections. Langenbecks Arch Surg. 2012;397:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Nesbakken A, Nygaard K, Lunde OC, Blücher J, Gjertsen Ø, Dullerud R. Anastomotic leak following mesorectal excision for rectal cancer: true incidence and diagnostic challenges. Colorectal Dis. 2005;7:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Tang CL, Seow-Choen F. Digital rectal examination compares favourably with conventional water-soluble contrast enema in the assessment of anastomotic healing after low rectal excision: a cohort study. Int J Colorectal Dis. 2005;20:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Dekker JW, Liefers GJ, de Mol van Otterloo JC, Putter H, Tollenaar RA. Predicting the risk of anastomotic leakage in left-sided colorectal surgery using a colon leakage score. J Surg Res. 2011;166:e27-e34. [PubMed] |
| 23. | den Dulk M, Noter SL, Hendriks ER, Brouwers MA, van der Vlies CH, Oostenbroek RJ, Menon AG, Steup WH, van de Velde CJ. Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol. 2009;35:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Warschkow R, Beutner U, Steffen T, Müller SA, Schmied BM, Güller U, Tarantino I. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Woeste G, Müller C, Bechstein WO, Wullstein C. Increased serum levels of C-reactive protein precede anastomotic leakage in colorectal surgery. World J Surg. 2010;34:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, McMillan DC. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19:4168-4177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Matthiessen P, Henriksson M, Hallböök O, Grunditz E, Norén B, Arbman G. Increase of serum C-reactive protein is an early indicator of subsequent symptomatic anastomotic leakage after anterior resection. Colorectal Dis. 2008;10:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Almeida AB, Faria G, Moreira H, Pinto-de-Sousa J, Correia-da-Silva P, Maia JC. Elevated serum C-reactive protein as a predictive factor for anastomotic leakage in colorectal surgery. Int J Surg. 2012;10:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Slotwiński R, Olszewski WL, Chaber A, Slodkowski M, Zaleska M, Krasnodebski IW. The soluble tumor necrosis factor receptor I is an early predictor of local infective complications after colorectal surgery. J Clin Immunol. 2002;22:289-296. [PubMed] |
| 30. | Iversen LH, Thomsen GH, Thorlacius-Ussing O. Systemic coagulation activation and anastomotic leakage after colorectal cancer surgery. Dis Colon Rectum. 1999;42:56-65. [PubMed] |
| 31. | Fouda E, El Nakeeb A, Magdy A, Hammad EA, Othman G, Farid M. Early detection of anastomotic leakage after elective low anterior resection. J Gastrointest Surg. 2011;15:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Uğraş B, Giriş M, Erbil Y, Gökpinar M, Citlak G, Işsever H, Bozbora A, Oztezcan S. Early prediction of anastomotic leakage after colorectal surgery by measuring peritoneal cytokines: prospective study. Int J Surg. 2008;6:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Matthiessen P, Strand I, Jansson K, Törnquist C, Andersson M, Rutegård J, Norgren L. Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer? Dis Colon Rectum. 2007;50:1918-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Herwig R, Glodny B, Kühle C, Schlüter B, Brinkmann OA, Strasser H, Senninger N, Winde G. Early identification of peritonitis by peritoneal cytokine measurement. Dis Colon Rectum. 2002;45:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Yamamoto T, Umegae S, Matsumoto K, Saniabadi AR. Peritoneal cytokines as early markers of peritonitis following surgery for colorectal carcinoma: a prospective study. Cytokine. 2011;53:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Bertram P, Junge K, Schachtrupp A, Götze C, Kunz D, Schumpelick V. Peritoneal release of TNFalpha and IL-6 after elective colorectal surgery and anastomotic leakage. J Invest Surg. 2003;16:65-69. [PubMed] |
| 37. | Ellebaek Pedersen M, Qvist N, Bisgaard C, Kelly U, Bernhard A, Møller Pedersen S. Peritoneal microdialysis. Early diagnosis of anastomotic leakage after low anterior resection for rectosigmoid cancer. Scand J Surg. 2009;98:148-154. [PubMed] |
| 38. | Tsujinaka S, Kawamura YJ, Konishi F, Maeda T, Mizokami K. Pelvic drainage for anterior resection revisited: use of drains in anastomotic leaks. ANZ J Surg. 2008;78:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 39. | Eckmann C, Kujath P, Kraus M, Schwandner O, Bruch HP, Shekarriz H. Therapeutic strategy for anastomotic leakage following low anterior resection. Viszeralchirurgie. 2005;40:17-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Millan M, García-Granero E, Flor B, García-Botello S, Lledo S. Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH. Dis Colon Rectum. 2006;49:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Junger W, Junger WG, Miller K, Bahrami S, Redl H, Schlag G, Moritz E. Early detection of anastomotic leaks after colorectal surgery by measuring endotoxin in the drainage fluid. Hepatogastroenterology. 1996;43:1523-1529. [PubMed] |
| 42. | Miller K, Arrer E, Leitner C. Early detection of anastomotic leaks after low anterior resection of the rectum. Dis Colon Rectum. 1996;39:1081-1085. [PubMed] |
| 43. | Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg. 1990;77:1095-1097. [PubMed] |
| 44. | Davies AH, Bartolo DC, Richards AE, Johnson CD, McC Mortensen NJ. Intra-operative air testing: an audit on rectal anastomosis. Ann R Coll Surg Engl. 1988;70:345-347. [PubMed] |
| 45. | Dixon AR, Holmes JT. Colorectal anastomotic integrity after anterior resection: is there a role for intraoperative testing? J R Coll Surg Edinb. 1991;36:35-36. [PubMed] |
| 46. | Gilbert JM, Trapnell JE. Intraoperative testing of the integrity of left-sided colorectal anastomoses: a technique of value to the surgeon in training. Ann R Coll Surg Engl. 1988;70:158-160. [PubMed] |
| 47. | Lazorthes F, Chiotassol P. Stapled colorectal anastomoses: peroperative integrity of the anastomosis and risk of postoperative leakage. Int J Colorectal Dis. 1986;1:96-98. [PubMed] |
| 48. | Ricciardi R, Roberts PL, Marcello PW, Hall JF, Read TE, Schoetz DJ. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg. 2009;144:407-411; discussion 411-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Schmidt O, Merkel S, Hohenberger W. Anastomotic leakage after low rectal stapler anastomosis: significance of intraoperative anastomotic testing. Eur J Surg Oncol. 2003;29:239-243. [PubMed] |
| 50. | Wheeler JM, Gilbert JM. Controlled intraoperative water testing of left-sided colorectal anastomoses: are ileostomies avoidable? Ann R Coll Surg Engl. 1999;81:105-108. [PubMed] |
| 51. | Yalin R, Aktan AO, Yeğen C, Döşlüoğlu H, Okboy N. Importance of testing stapled rectal anastomoses with air. Eur J Surg. 1993;159:49-51. [PubMed] |
| 52. | Ishihara S, Watanabe T, Nagawa H. Intraoperative colonoscopy for stapled anastomosis in colorectal surgery. Surg Today. 2008;38:1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Smith S, McGeehin W, Kozol RA, Giles D. The efficacy of intraoperative methylene blue enemas to assess the integrity of a colonic anastomosis. BMC Surg. 2007;7:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Griffith CD, Hardcastle JD. Intraoperative testing of anastomotic integrity after stapled anterior resection for cancer. J R Coll Surg Edinb. 1990;35:106-108. [PubMed] |
| 55. | Sakanoue Y, Nakao K, Shoji Y, Yanagi H, Kusunoki M, Utsunomiya J. Intraoperative colonoscopy. Surg Endosc. 1993;7:84-87. [PubMed] |
| 56. | Lanthaler M, Biebl M, Mittermair R, Ofner D, Nehoda H. Intraoperative colonoscopy for anastomosis assessment in laparoscopically assisted left-sided colon resection: is it worthwhile? J Laparoendosc Adv Surg Tech A. 2008;18:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Li VK, Wexner SD, Pulido N, Wang H, Jin HY, Weiss EG, Nogeuras JJ, Sands DR. Use of routine intraoperative endoscopy in elective laparoscopic colorectal surgery: can it further avoid anastomotic failure? Surg Endosc. 2009;23:2459-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Shamiyeh A, Szabo K, Ulf Wayand W, Zehetner J. Intraoperative endoscopy for the assessment of circular-stapled anastomosis in laparoscopic colon surgery. Surg Laparosc Endosc Percutan Tech. 2012;22:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Ambrosetti P, Robert J, Mathey P, Rohner A. Left-sided colon and colorectal anastomoses: Doppler ultrasound as an aid to assess bowel vascularization. A prospective evaluation of 200 consecutive elective cases. Int J Colorectal Dis. 1994;9:211-214. [PubMed] |
| 60. | Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43:76-82. [PubMed] |
| 61. | Kudszus S, Roesel C, Schachtrupp A, Höer JJ. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg. 2010;395:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 62. | Hirano Y, Omura K, Tatsuzawa Y, Shimizu J, Kawaura Y, Watanabe G. Tissue oxygen saturation during colorectal surgery measured by near-infrared spectroscopy: pilot study to predict anastomotic complications. World J Surg. 2006;30:457-461. [PubMed] |
| 63. | Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis. 2010;12:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Novell JR, Lewis AA. Peroperative observation of marginal artery bleeding: a predictor of anastomotic leakage. Br J Surg. 1990;77:137-138. [PubMed] |
| 65. | Sheridan WG, Lowndes RH, Young HL. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;30:867-871. [PubMed] |
| 66. | Akyol AM, McGregor JR, Galloway DJ, George WD. Early postoperative contrast radiology in the assessment of colorectal anastomotic integrity. Int J Colorectal Dis. 1992;7:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Doeksen A, Tanis PJ, Wüst AF, Vrouenraets BC, van Lanschot JJ, van Tets WF. Radiological evaluation of colorectal anastomoses. Int J Colorectal Dis. 2008;23:863-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | DuBrow RA, David CL, Curley SA. Anastomotic leaks after low anterior resection for rectal carcinoma: evaluation with CT and barium enema. AJR Am J Roentgenol. 1995;165:567-571. [PubMed] |
| 69. | Haynes IG, Goldman M, Silverman SH, Alexander-Williams J, Keighley MR. Water-soluble contrast enema after colonic anastomosis. Lancet. 1986;1:675-676. [PubMed] |
| 70. | Hoffmann J, Jensen RH, Shokouh-Amiri MH, Damm P. Clinical value of water-soluble contrast enema in assessing the integrity of left colonic anastomoses. J R Coll Surg Edinb. 1988;33:23-24. [PubMed] |
| 71. | Kalady MF, Mantyh CR, Petrofski J, Ludwig KA. Routine contrast imaging of low pelvic anastomosis prior to closure of defunctioning ileostomy: is it necessary? J Gastrointest Surg. 2008;12:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Markham NI, Greatorex RA, Everett WG. The value and significance of the limited barium enema examination following restorative resection for carcinoma of the rectum. Ann R Coll Surg Engl. 1987;69:116-118. [PubMed] |
| 73. | Nicksa GA, Dring RV, Johnson KH, Sardella WV, Vignati PV, Cohen JL. Anastomotic leaks: what is the best diagnostic imaging study? Dis Colon Rectum. 2007;50:197-203. [PubMed] |
| 74. | Severini A, Civelli EM, Uslenghi E, Cozzi G, Salvetti M, Milella M, Gallino G, Bonfanti G, Belli F, Leo E. Diagnostic and interventional radiology in the post-operative period and follow-up of patients after rectal resection with coloanal anastomosis. Eur Radiol. 2000;10:1101-1105. [PubMed] |
| 75. | Gouya H, Oudjit A, Leconte M, Coste J, Vignaux O, Dousset B, Legmann P. CT antegrade colonography to assess proctectomy and temporary diverting ileostomy complications before early ileostomy takedown in patients with low rectal endometriosis. AJR Am J Roentgenol. 2012;198:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Tang CL, Yeong KY, Nyam DCNK, Eu KW, Ho YH, Leong AFPK, Tsang CBS, Seow-Choen F. Postoperative intra-abdominal free gas after open colorectal resection. Dis Colon Rectum. 2000;43:1116-1120. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Eckmann C, Kujath P, Schiedeck TH, Shekarriz H, Bruch HP. Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis. 2004;19:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 78. | Power N, Atri M, Ryan S, Haddad R, Smith A. CT assessment of anastomotic bowel leak. Clin Radiol. 2007;62:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Williams CE, Makin CA, Reeve RG, Ellenbogen SB. Over-utilisation of radiography in the assessment of stapled colonic anastomoses. Eur J Radiol. 1991;12:35-37. [PubMed] |
| 80. | Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873. [PubMed] |
| 81. | Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 82. | Merkel S, Wang WY, Schmidt O, Dworak O, Wittekind C, Hohenberger W, Hermanek P. Locoregional recurrence in patients with anastomotic leakage after anterior resection for rectal carcinoma. Colorectal Dis. 2001;3:154-160. [PubMed] |
| 83. | den Dulk M, Marijnen CA, Collette L, Putter H, Påhlman L, Folkesson J, Bosset JF, Rödel C, Bujko K, van de Velde CJ. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 84. | van Ramshorst GH, Vos MC, den Hartog D, Hop WC, Jeekel J, Hovius SE, Lange JF. A comparative assessment of surgeons’ tracking methods for surgical site infections. Surg Infect (Larchmt). 2013;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |